www.acsprf.org
Reports: ND750863-ND7: Ion gels from block copolymers composed of liquid crystalline units and brush-like moieties in ionic liquids
Rajeswari Kasi, PhD , University of Connecticut
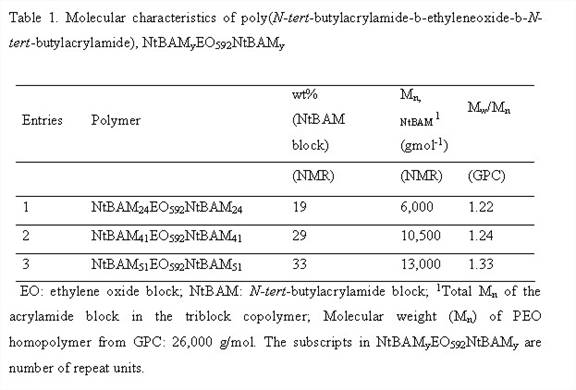
This project has developed in two different directions: (1) design and preparation of ion-gels from triblock copolymers containing PEO and non-liquid crystalline polyacrylamides and (2) synthesis and characterization of norbornene-based diblock copolymers bearing semicrystalline PEO brushes and liquid crystalline cholesterol side chains. At this juncture, project 1, for which some PRF funds was used, is near-completion and I will focus this annual report on this project. We have synthesized block copolymers that will be used in project 2 and characterization of these materials is currently under investigation.
Project 1:
Introduction and general goals
Ion-gels consisting of a swollen polymeric network in an ionic liquid (IL) constitute a promising class of solid state electrolytes owing to their good mechanical properties and high ionic conductivity which can be used in Li-ion batteries, electrochemical devices, sensors, electromechanical actuators, gas separation membranes, and dye-sensitized solar cells. Most of the studies on ion-gels derived from polymers are based on (1) doping of ILs with polymers, (2) in situ polymerization (or cross-linking) of vinyl monomers in ILs, (3) self-assembly of ABA triblock copolymer in a B-block compatible IL. Despite the availability of thousands of ILs, to our knowledge, there are only three reports on ion-gels based on gelation of two ILs ([BMIM][PF6] and [EMI][TFSI]), obtained from the self-assembly of block copolymers. Most of these studies are confined to gelation and ionic conductivity at one composition, except one in which the effect of concentration, temperature, and polymer identity on the ionic and viscoelastic motions of ion-gels is studied using PS-PEO-PS and PS-PMMA-PS triblock copolymer in 1-ethyl-3-methylimidazolium bis(trifluoromethyl-sulfonyl)imide, [EMI][TFSI].
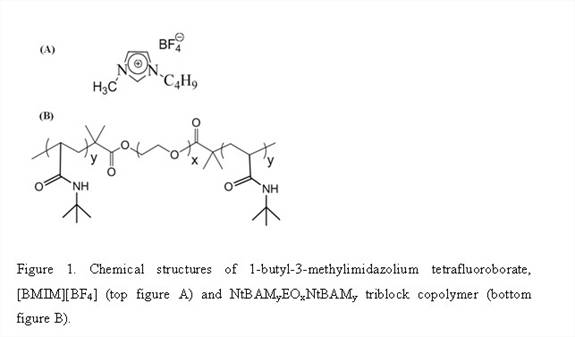
It is widely acknowledged that systematic and comprehensive mechanical and electrical property evaluation of ion-gels via self-assembly of block copolymers is desirable. The objective of this work is to demonstrate the gelation of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) using poly(N-tert-butylacrylamide-b-ethylene oxide-b-N-tert-butylacrylamide) (NtBAMyEOxNtBAMy) triblock copolymer through the process of self-assembly. [BMIM][BF4] has one of the highest conductivities observed in the general class of ILs and based on task-specific properties, it stands out as a good electrolyte solvent for lithium ion batteries due to its high ionic conductivity, Li-salt solubility at room temperature and broad electrochemical window with high anodic stability (5 V) compared to other ionic liquids. The only pertinent paper on [BMIM][BF4] ion-gels using polymers is by in situ copolymerization of acrylonitrile (AN), methyl methacrylate (MMA), poly (ethylene glycol) methyl ether methacrylate (PEGMEMA) in [BMIM][BF4]. Ion-gels from these random copolymers are obtained at high concentrations (≥ 30 wt%) with conductivities significantly lower than the bulk [BMIM][BF4]. Nevertheless, there have been neither reports on the gelation of [BMIM][BF4] by macromolecular self-assembly nor mechanical, thermal and conductivity investigations of the corresponding ion-gels. Thus the current study focuses on designing highly conductive polymeric ion-gels in [BMIM][BF4] at lower concentrations through the self-assembly of block copolymer which are promising candidates for solid-state electrolyte applications.
Results and discussions
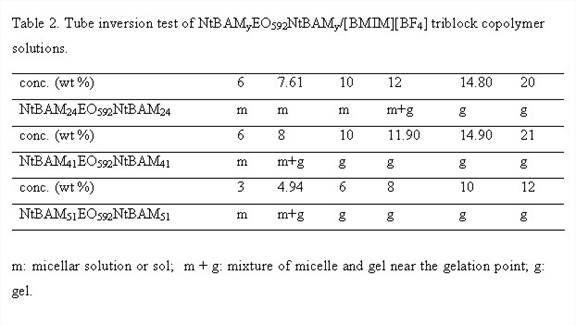
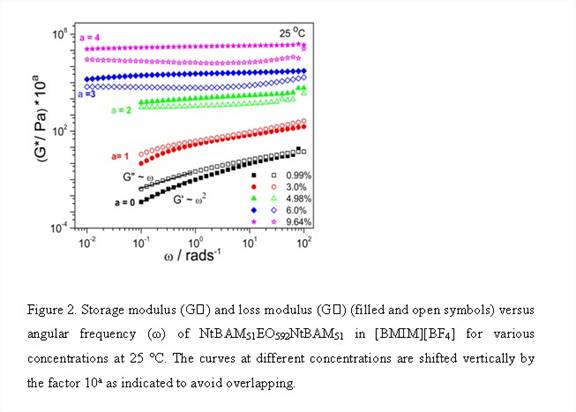
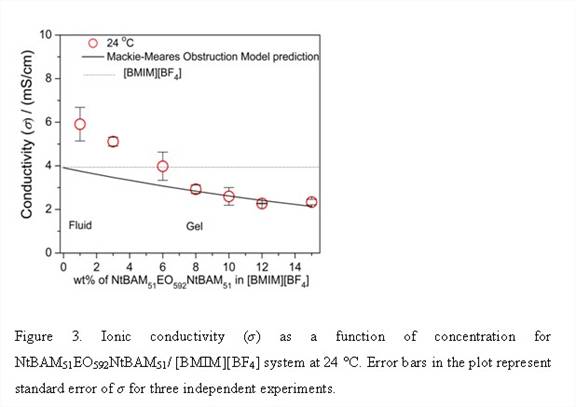
Firstly, we synthesized and characterized triblock copolymer NtBAMyEOxNtBAMy (where EO denotes ethylene oxide block, NtBAM denotes N-tert-butylacrylamide block and x, y are number of repeat units) by reversible addition-fragmentation chain transfer (RAFT) technique, Figure 1 and Table 1. [BMIM][BF4] known as a good solvent for polyethylene oxide block forms ion-gels by non-covalent intermolecular aggregation of the triblock copolymer, Table 2. Thereafter we investigated the frequency- and concentration dependence of the linear viscoelastic moduli of the triblock copolymer solutions and gels at different concentrations and effect of acrylamide (NtBAM) block length (or copolymer composition) on the gelation characteristics, Figure 2. Finally, we investigated frequency and concentration dependence of the ionic conductivity (σ) of the ion-gels, Figure 3.
Summary of results
We describe a new set of ion-gels prepared via self-assembly of poly(N-tert-butylacrylamide-b-ethyleneoxide-b-N-tert-butylacrylamide) triblock copolymer in a room temperature ionic liquid (IL), 1-butyl-3-methylimidazolium tetrafluoroborate, [BMIM][BF4]. At moderately low concentrations of 6 wt%, we demonstrate the formation of strong and thermoreversible ion-gel with high gelation temperature (Tgel ~45 °C) and ionic conductivities similar to bulk [BMIM][BF4]. This meets both the crucial requirement of mechanical integrity and high ionic conductivity (similar to IL) of solid-state electrolytes required for target applications. It further gives us a handle of copolymer concentration or solvophobic block length to tune mechanical and conductivity properties so as to meet the design specific criterions for various electrochemical applications. Conductivity studies on these gels have shown moderate affect due to structuring soft materials as compared to bulk IL and thus are good candidates for electrolyte applications. The proposed ionic liquid gels obtained via gelation of [BMIM][BF4] utilizing a triblock copolymer is an alternative and attractive way for development of novel solid state electrolytes.