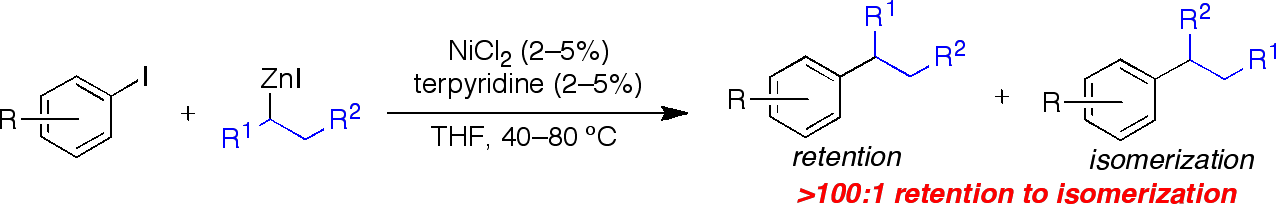www.acsprf.org
Reports: DNI150307-DNI1: Fluorophobic Effects in Homogeneous Catalysis
Mark R. Biscoe, PhD , City University of New York (City College)
Between the submission date for the original grantapplication and the date on which the grant was awarded, the general directionof research in my laboratory has evolved. Initially, we proposed to explore the effects of fluorophobicity ontransition metal-catalyzed reactions. The goal of this proposed research was the development of a novelstrategy to generate value added materials from resources derived frompetroleum feedstocks. The goal ofdeveloping new, useful methods to rapidly generate value added materialsremains the general goal of our research program. However, our approach towards this end now involves thedevelopment of methods (fluorous/non-fluorous) to produce non-racemic moleculesusing transition metal-catalyzed carbon-carbon bond-forming reactions thatemploy configurationally-stable, optically-active nucleophiles. By establishing the stereogenic centerprior to the formation of the final desired bond, the rapid preparation ofdiverse libraries of single-enantiomer molecules would be achievable.
Thedevelopment of a general protocol for the direct cross-coupling of an arylhalide and a racemic secondary organometallic nucleophile is required prior to thedevelopment of extensions that employ optically-active organometallic nucleophiles,so we have first concentrated on this area. Only two methods, each employing palladium catalysis, havebeen previously reported for the transition metal-catalyzed cross-coupling ofaryl halides and unactivated, acyclic secondary organometallic nucleophiles.1Unfortunately, these palladium-catalyzed systems suffer from varying degrees ofnucleophile isomerization during the catalytic cycle. It is essentialthat this isomerization process be eliminated or kept to an absolute minimum tomaintain the integrity of the optically-active center of the secondarynucleophile. With this in mind, we decided to explore nickel catalysis as analternative to palladium catalysis in our initial studies on thesecross-coupling reactions.
Overthe past year, our group has developed a reliable and general method to achievethe Ni-catalyzed cross-coupling of unactivated secondary alkyl zinc halidenucleophiles and aryl iodides.2 While palladium-catalyzed systems may still prove effectivein general catalytic systems for cross-coupling reactions involving secondaryalkyl nucleophiles, we have been able to demonstrate the superiority of nickelcatalysis in these systems. Usinga terpyridine ligand, we are able to achieve an unprecedented level ofretention in the secondary cross-coupling reactions of secondary alkyl zinc iodides and aryl iodides (Figure 2). We see only nominal evidence of anisomerization product (> 500 retention : 1 isomerization), which is animprovement of more than a factor of ten compared to the best ratio achieved inthe previously reported palladium-based system.1 This method is completely independentof the electronic characteristics of the aryl iodide employed. Previous, Pd-catalyzed methods requiredthe use of electronically activated (electron-deficient) or stericallyactivated (bearing a ortho-substituent) electrophiles in order to avoidisomerization pathways. Different secondary alkylzinc nucleophiles can be employed in thisNi-catalyzed cross-coupling reaction in a general fashion with isomericretention. In addition toperforming cross-coupling reactions with acyclic alkylzinc nucleophiles such asi-PrZnI and s-BuZnI, we have successfully employed a secondary alkylzinc reagent bearing anester, as well as a secondary alkylzinc reagent with a-branching on both alkyl substituents. These reactions occurred in good to excellent yields withoutdetectable isomerization of the nucleophile.
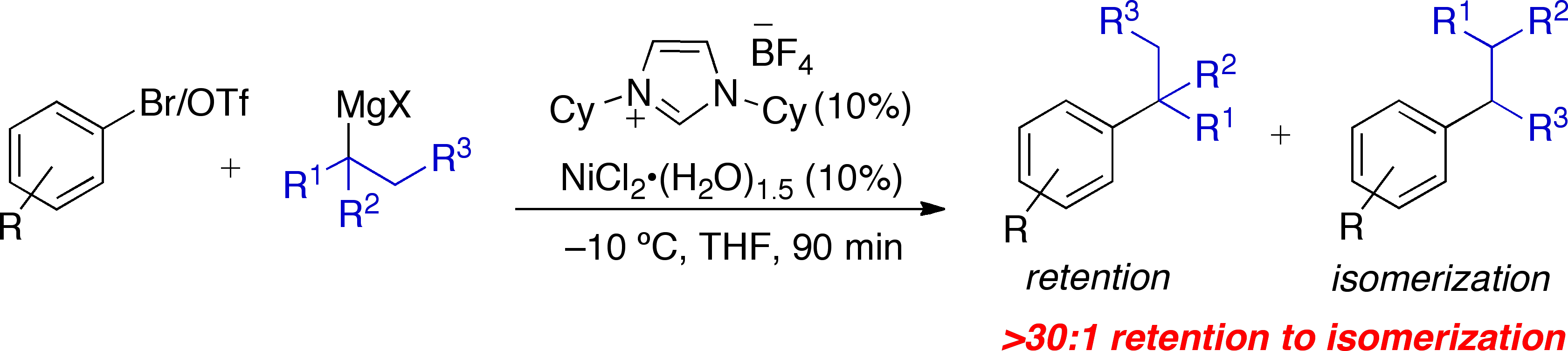
Inan attempt to build upon this work, we also investigated the possibility that anickel-based catalytic system could support the direct cross-coupling of tertiaryalkyl nucleophiles and aryl halidesto form aryl-substituted quaternary centers. Carrying out such a reaction in an effective and efficientmanner is indeed a significant challenge; the procedure would need to supporttransmetallation of a sterically hindered tertiary nucleophile, while additionally promoting facile oxidative additionand reductive elimination such that b-hydride eliminationis averted. The few existingreports of Pd- and Ni-catalyzed cross-coupling reactions employing tertiarynucleophiles have led to the exclusive formation of isomerized cross-couplingproducts due to rapid b-hydride elimination.3 We are excited to report that wehave successfully developed a Ni-catalyzed process for the cross-coupling oftertiary alkyl nucleophiles and aryl bromides, employing N-heterocylcic carbene(NHC) as supporting ligands.4 This process is extremely general fora wide range of electrophiles and generally occurs witha ratio of retention to isomerization >30:1. The same procedure also accommodates the use of aryltriflates, vinyl chlorides, and vinyl bromides as the electrophilic component. Inorder to verify that tertiary alkylmagnesium halides could be employed in thisNi-catalyzed cross-coupling process in a general fashion, we performedcross-coupling reactions with nucleophiles other than t-BuMgCl. The presence of a mono-a-branched nucleophile (e.g., t-amylMgCl) was well tolerated in these reactions and resulted innegligible isomerization.
Thesecured funding from the ACS Petroleum Research Fund has facilitated thedevelopment of the groundwork for the direct transition metal-catalyzedcross-coupling reaction of optically-active main group nucleophiles and arylelectrophiles. General methodsthat enable cross-coupling reactions using racemic acyclic secondary andacyclic tertiary nucleophiles have been developed. We are currently exploring the use of optically activenucleophiles in place of the corresponding racemic mixtures. We expect that methods that enable therapid creation of optically active molecules will have immediate application inmedicinal and material sciences. This work will support future solicitations of funding from federal andprivate agencies. Students participatingin this research have gained valuable experience in the field of organometalliccatalysis. Such catalytic methodsare routinely employed in the fields of pharmaceutical science and drugdiscovery.
REFERENCES
1) a) Dreher, S. D.;Dormer, P. G.; Sandrock, D. L.; Molander, G. A. J. Am. Chem. Soc. 2008, 130, 9257. b) Han, C.; Buchwald, S. L. J. Am. Chem. Soc. 2009, 131, 7532.
2) Joshi-Pangu, A.; Ganesh,M.; Biscoe, M. R. Org. Lett. 2011, 13,1218.
3) a) Luo, X.; Zhang, H.;Duan, H.; Liu, Q.; Zhu, L.; Zhang, T.; Lei, A. Org. Lett. 2007, 9, 4571. b) Breitenfeld,J.; Vechorkin, O.; Corminboeuf, C.; Scopelliti, R.; Hu, X. Organometallics 2010, 29, 3686.
4) Joshi-Pangu, A.; Wang,C.-Y.; Biscoe, M. R. J. Am.Chem. Soc. 2011, 133,8478.