www.acsprf.org
Reports: DNI1049007-DNI10: Electroceramic Materials for High-Purity Hydrogen Extraction from Liquid Hydrocarbon Fuels: Fundamental Investigations of Coupled Electrochemical and Catalytic Phenomena
Benjamin A. Wilhite , University of Connecticut
Summary
During the past funding period, substantial information regarding the impact of perovskite composition (dopant amount, A:B cation ratio) upon catalytic activity, materials stability under moist reducing conditions and electrochemical activity has been collected. Stability analysis of these materials indicates the presence of a complex decomposition pathway under moist reducing environments that may produce highly active catalytic structures. The catalytic activity of these nanoporous ceria-zirconia powders is currently being explored through a new collaboration with Dr. Graham Hutchenson (Cardiff, UK). Stable perovskites are confirmed for compositions of (BaCe0.25Co0.05Zr0.703-a), with initial electrochemical analysis indicating promising mixed protonic-ionic conductivity.
Material Synthesis
BCZC was synthesized via solid-state reaction from oxide powders in order to directly control the A:B cation ratio and Co doping levels. Samples spanning a range of dopant level and A:B cation ratio were produced to understand the influence of composition upon electrochemical stability and catalytic activity. Milled powder mixtures were calcined in air at 1550oC for 4 hours to achieve uniform calcination of the precursor powder. A summary of compositions is provided in Table I, below.
Table 1: Different BCZC compositions selected for electrochemical, catalytic analysis
Material
| Formula
| Co doping (mol %)
| A:B ratio
|
BCZ
| BaCe 0.25 Zr 0.75 O 3-d | 0
| 1.00
|
BCZC5
| BaCe0.25Zr0.70 Co 0.05 O3- d
| 5
| 1.00
|
BCZC10
| BaCe0.25Zr0.65 Co 0.10 O3- d
| 10
| 1.00
|
BCZC15
| BaCe0.25Zr0.60 Co 0.15 O3- d
| 15
| 1.00
|
BCZ55C
| BaCe0.25Zr0.55 Co 0.15 O3- d
| 15
| 1.05
|
BCZ50C
| BaCe 0.25Zr0.50 Co 0.15 O3- d
| 15
| 1.11
|
BCZ45C
| BaCe0.25Zr0.45 Co 0.15 O3- d
| 15
| 1.18
|
Electrochemical Analysis
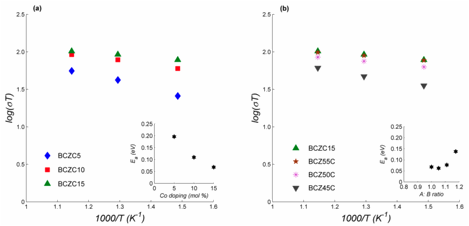
Electrochemical Impedance spectroscopy was carried out at 400 – 600oC under dry inert, moist inert and moist H2 environments to investigate bulk electrical conductivity of each material. Analysis of electrical conductivity over the range of compositions studied indicates that cobalt-doping increases the electrical conductivity, while reducing the associated activation energy for charge transport. Increasing the A:B cation ratio increases activation energy for transport while reducing electrical conductivity (summarized in Figure 1). These results may be explained in terms of the formation of a cermet-type microstructure (confirmed in previous report) in which a metal-rich integranular phase evolves at elevated temperatures (Suresh et al., 2010; 2011). Upon completion of electrochemical analysis, all pellets were stored under dry inert conditions for 40 days and inspected for mechanical stability. All pellets except BCZC5 and BCZC10 displayed substantial degradation and cracking due to in-situ hydrolysis and metal reduction.
Figure 1: Summary of electrical conductivity and activation energy under moist H2 environment as function of materials composition; (a) increasing Co dopant level, A:B cation ratio of unity; (b) increase A:B cation ratio from unity, Co dopant level of 15 mol%.
Catalytic Evaluation of Materials
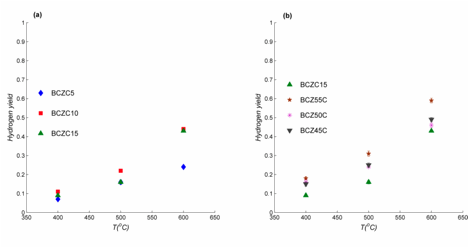
Sintered disks of each material were crushed and placed in a differential-packed bed reactor for evaluating catalytic activity for methanol partial oxidation. Dilute mixtures (4% MeOH, 2% O2) were passed over the catalyst bed at sufficient flowrates to maintain ~30-50% conversion and effluent compositions monitored by gas chromatography. Reaction temperatures of 400 – 600C are selected based upon expected electrochemical cell operating temperatures. In all cases, reaction rates over cobalt-doped materials are approximately an order-of-magnitude greater than values reported in the literature for ZrO2. Results indicate that the maximum Co doping level (15mol%) achieved highest hydrogen product yields, while an optimal A:B cation ratio of 1.1:1 resulted in the highest hydrogen yield of all materials.
Figure 2: Hydrogen yields for crushed sintered disks of each material for methanol partial oxidation; (a) varying Co doping level at A:B cation ratio of unity; (b) varying A:B cation ratio at fixed Co doping of 15mol%.
New Discovery: Controlled formation of nanoporous oxide microstructures.
During the past funding period, the PI has confirmed a unique in-situ mechanism for creating nano-porous powders comprised of semiconductor CeO2-ZrO2 oxides. Under reducing environments, excess transition-metal dopant may reduce to ground-state (exolving and collecting at the grain boundaries), leaving behind B-cation site vacancies (Eqn I). Additionally, non-stoichiometry of the perovskite (A:B cation ratios > 1) results in B-cation site vacancies. These vacancies can drive the exolution of Barium ions to form barium oxide at the grain boundaries (Eqn II). Conversely, these vacancies may react with dissolved protons (hydroxide ions within the lattice) and lattice barium to directly hydrolyze barium (Eqn III). This latter mechanism produces a nanoporous CeO2-ZrO2 grain system which has been confirmed during the past funding period (Figure 3). This mechanism confirms the high protonic conductivity of the material. The resulting highly porous powders are expected to yield valuable catalytic structures; this hypothesis is currently being investigated through a new collaboration with Dr. Graham Hutchenson (Cardiff, UK). Samples of the material shown in Figure 3 have been shipped to Dr. Hutchenson's lab, and will be deposited with Au nanoclusters and evaluated as low-temperature water-gas-shift catalysts.
Figure 3: Evolution of nanoporous CeO2-ZrO2 under controlled reduction of BaCe0.25Co0.15Zr0.4O3. (a) TEM image of an FIB cross-section of as-sintered cell; (b) TEM images of an FIB cross-section of sintered cell after 24hr exposure to 10% H2 / 10% H2O at 400oC; (c) diffraction analysis of TEM images confirm presence of CeO2 and ZrO2 phases, absence of BaO and CoO.
Future Work
The PI has identified compositions suitable for prolonged electrochemical analysis via impedance spectroscopy and concentration-cell studies. During the next six months, the PI will oversee the electrochemical analysis of BaCe0.25Co0.05Zr0.7O3-a button-cells via carrying out a sequence of (i) transient electrical conductivity relaxation analysis followed by (ii) electrochemical impedance spectroscopy under varying oxygen, moisture and moist hydrogen partial pressures at 600oC. This analysis will fully characterize stable compositions identified through the work-to-date. Finally, concentration-cell tests will be performed to investigate coupled electrochemical reaction and separation using methanol partial oxidation and/or water-gas-shift, based upon materials stability under each reaction environment.