www.acsprf.org
Reports: UR149441-UR1: Gold(I)-Catalyzed Aromatic Claisen Rearrangements
James R. Vyvyan, PhD , Western Washington University
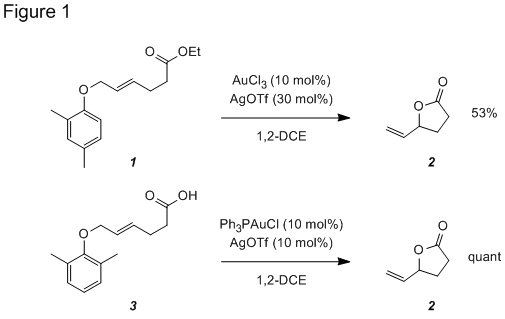
A significant portion of year 2 was used to explore the Au-catalyzed formation of substituted lactones and tetrahydropyrans. Last year we reported the conversion of ester 1 to lactone 2 using a Au(III) catalyst (Figure 1). Substrate 3 with both ortho-positions blocked to prevent a competing Claisen rearrangement and a carboxylic acid replacing the ester, we found that lactone 2 was produced in quantitative yield using Ph3PAuOTf catalyst.
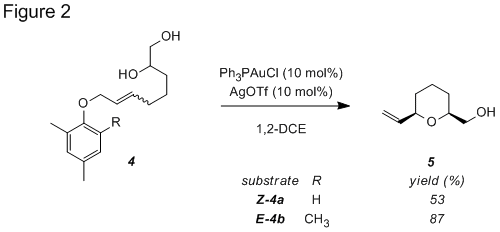
Similarly, blocking both ortho-positions of diol 4 resulted in an improved yield of tetrahydropyran 5 (Figure 2). In each case the tetrahydropyran product was exclusively of the cis-relative configuration.
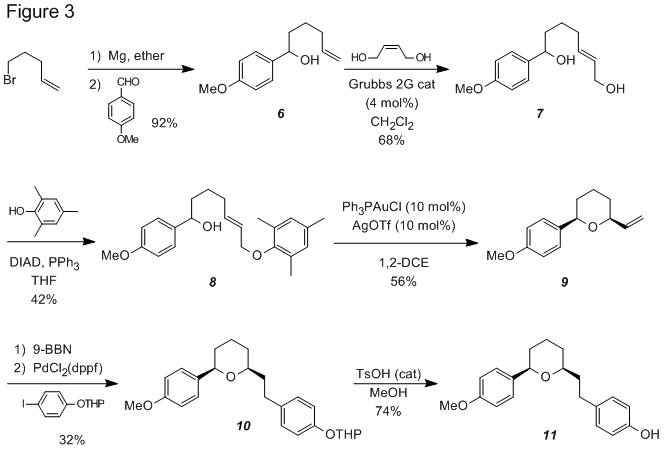
Capitalizing on the high yield and stereospecificity of this transformation, we completed a synthesis of (±)-centrolobine using this cyclization reaction (Figure 3). Addition of 4-pentenylmagnesium bromide to anisaldehyde resulted in secondary alcohol 6 in good yield. Grubb's second-generation catalyst was used to promote an olefin cross metathesis between 6 and cis-2-buten-1,4-diol to produce allylic alcohol 7. Mitsunobu etherification of 7 with 2,4,6-trimethylphenol gave the allyl aryl ether 8 which cyclized to form tetrahydropyran 9 upon treatment with Ph3PAuOTf. A B-alkyl Suzuki coupling of 9 with the THP ether of 4-iodophenol produced 10 which was deprotected to produce (±)-centrolobine (11).
During the course of many, but not all, of our gold-catalyzed rearrangement reactions, we observed the formation of a gold film on the surface of the reaction vessel (Figure 4). Further investigation revealed that the film consists of aggregated gold nanoparticles. The film has been characterized with AFM, XRD, and ICP-MS to date. When the initial reaction solution is removed from the reaction vessel, the gold film rinsed with fresh solvent, and additional substrate added, the rearrangement reaction proceeds quickly. This indicates to us that the gold film is capable of catalyzing the rearrangement reaction and a soluble gold(I) or gold(III) complex is not required for catalysis. Current work is further exploring the nature of the gold film and its catalytic properties.
Figure 4. Gold film on the surface of an NMR tube and AFM image of the film.