www.acsprf.org
Reports: UNI349510-UNI3: Chirality Recognition by Macrocyclic and Multi-Functional Metal Complexes
J. Frantz Folmer-Andersen, PhD , State University of New York, College at New Palz
Summary of work completedduring grant period
The aim of this project was to synthesize a series ofchiral macrocyclic metal complexes that are capable of enantioselectivelybinding small molecule guests within their internal cavities. Our effortsfocused primarily on trans-1,2-diaminocyclohexane(DACH)-containing macrocycles, in which the DACH subunit serves both as abidentate ligand, and as a source of chirality.
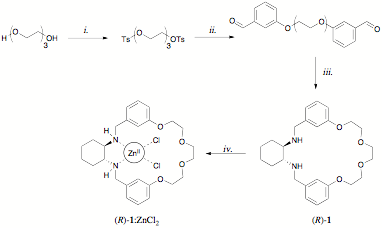
During the summer of 2009, Chemistrymajor Tyler Moore developed the synthesis of the C2-symmetric macrocycle (R)-1 asa prototype ligand, according to the route shown in Scheme 1. 1H NMRtitration experiments indicated that the complex (R)-1:ZnCl2was cleanly formed by treatmentof (R)-1 with one equivalent of ZnCl2 in methanol,and the identity of the complex was confirmed by X-ray crystallography. UV-vistitrations suggest that (R)-1 interacts very weakly with Cu(OTf)2. Itwas also noted that (R)-1 causes significant non-equivalence of the a-1H NMR resonance of racemic mandelic acidin CDCl3, suggesting that such compounds may have potential aschiral shift reagents.
Scheme1: i. TsCl, KOH, CH2Cl2; ii. m-hybroxybenzaldehyde,K2CO3, NaI, MeCN; iii. (a) (R,R)-1,2-diaminocyclohexane, MeOH (b) NaBH4; iv. ZnCl2, MeOH.
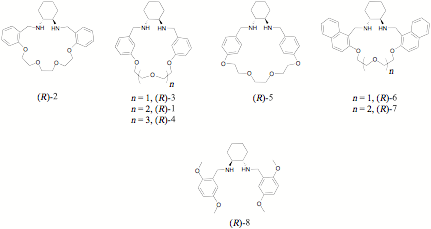
Overthe 2009-2010 academic year and the Summer of 2010, Chemistry major DavidAtwood prepared the library of structural analogs of (R)-1shown in Figure 1 by routes similar to that shown in Scheme 1. X-ray crystalstructures were obtained of the free-based forms of (R)-1, (R)-3, (R)-4 and(R)-7. Interestingly, only the meta-linked macrocycles (R)-1, (R)-3,and (R)-4 were observed by 1H NMR to formsymmetrical and well-defined 1:1 complexes with ZnCl2 in methanol.Severe broadening of the 1H NMR spectrum and precipitation wereobserved when the other compounds were treated with ZnCl2.
Figure 1.
The ability of the compounds shown in Figure 1 todifferentiate the enantiomers of mandelic acid (MA) was studied by 1HNMR during the Summer of 2010 by Biology major Thomas Quinn (supported by aninternal grant). Compounds (R)-1,(R)-4,and (R)-7 were found to be the most effective indifferentiating the enantiomers of MA and a variety of arene-substitutedderivatives. Both small-ring macrocycles ((R)-3,and (R)-6) were virtually ineffective, whereas the ortho- and para-linked macrocycles ((R)-2, and(R)-5)provided similar non-equivalencies to the acyclic control compound (R)-8.Binding titrations performed on single enantiomers of MA revealed that, innearly all cases the macrocycles interact with MA through a simultaneous 1:1and 2:1 (MA:macrocycle) binding model, and that the observed enantioselectivityarises from the 2:1 complexes. For five of the macrocycles, the bindingtitration data were fitted to obtain apparent 2:1 binding constants to each MAenantiomer using the computer program NMRtit HGG.
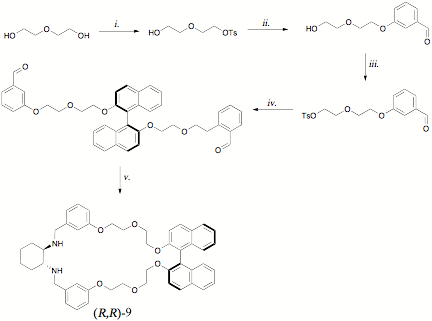
Efforts to incorporate 1,1«-bi-2-naphol (BINOL) as amacrocyclic subunit were initiated by David Atwood in the Summer of 2010, andcarried forward by Chemistry major Shaun Ben-Ari during the 2010-2011 academicyear and the Summer of 2011. In addition to providing for the possibility offluorescence monitoring of binding processes, BINOL imparts structural rigidityand an additional stereogenic center. Mr. Atwood's initial approach centered onthe desymmetrization of diethylene glycol as shown in Scheme 2. While thissequence provided the desired macrocycle (R,R)-9, the yield for the final step was < 10% becauseof difficulties with chromatographic purification. Mr. Ben-Ari's later attemptsto optimize this separation improved the process only marginally, and in lightof the poor performance of (R,R)-9 asa chiral shift reagent toward mandelic acid, we embarked on the alternative synthesis shown in Scheme3.
Scheme2: i. TsCl, TEA, CH2Cl2; ii. m-hybroxybenzaldehyde,K2CO3, NaI, MeCN; iii. TsCl, TEA, CH2Cl2; iv. (R)-BINOL,K2CO3, NaI, DMF v. (a) (R,R)-1,2-diaminocyclohexane, CH2Cl2MeOH (b) NaBH4.
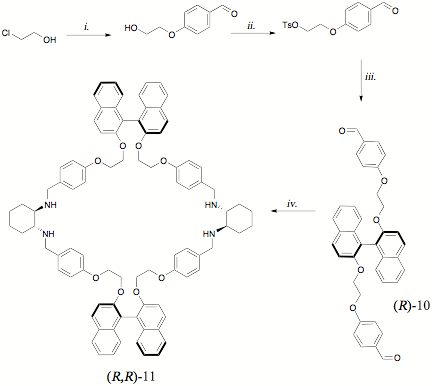
While the use of b-chloroethanol as the starting material in Scheme 3 simplifiedthe synthesis of the BINOL-containing di-aldehyde (R)-10,the shorter flexible linker was expected to yield a [1+1] macrocycle that wasconsiderably smaller than (R,R)-9,and so the para-linked phenylenespacer was employed to partially compensate. Surprisingly, after mixing (R)-10and (R)-DACH in acetonitrile forseveral days, a precipitate was formed that was determined to be thecorresponding tetra-imine of (R,R)-11.The tetra-imine was reduced quantitatively to give analytically pure [2+2] macrocyclizationproduct in nearly 80% yield (on a multi-gram scale). This was accomplishedwithout resorting to chromatographic separation in the final step, which istypically the most difficult aspect of the synthesis of this class ofcompounds. The four stereoisomers of 11 (excluding those derived from meso-DACH) have been prepared, and their use as enantioselectivefluorescence sensors for chiral carboxylic acid derivatives is currently underinvestigation.
Scheme3: i. p-hybroxybenzaldehyde, KOH, H2O; ii. TsCl, TEA, CH2Cl2; iii. (R)-BINOL,K2CO3, NaI, MeCN iv. (a) (R,R)-1,2-diaminocyclohexane, MeCN (b) NaBH4,MeOH.
Studentinvolvement: Four students havedirectly benefited from ACS-PRF support of this work. The support providedsummer research fellowships to two students (Moore and Atwood), in addition tochemicals and supplies that greatly facilitated the work of two others (Quinnand Ben-Ari). A manuscript has been prepared detailing the use of themacrocycles from Figure 1 as chiral shift reagents for mandelic acidderivatives on which Quinn, Atwood, and Moore are co-authors. We are currentlyin the process of preparing a manuscript describing the synthesis of (R,R)-11 and its use as an enantioselective fluorescencesensor, on which Ben-Ari will be a co-author.
Othercollaborators: ACS-PRF support ofthis work encouraged the establishment of professional contacts which enableduse of instrumentation that was otherwise unavailable. Four X-ray crystalstructures were obtained in collaboration with Professor Joseph Tanski, andtwelve high-resolution were obtained in collaboration with Professor TeresaGarrett. Both Tanski and Garrett are at Vassar College.