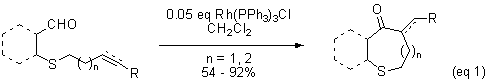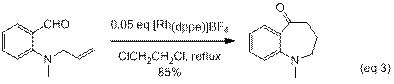www.acsprf.org
Reports: UR149342-UR1: Rhodium(I)-Catalyzed Hydroacylation Promoted by Chelating Amines
Holly D. Bendorf, PhD , Lycoming College
While there are numerous reports in the literature of rhodium-catalyzed heteroatom-directed intermolecular hydrolacylation, relatively few examples of the analogous intramolecular hydroacylation exist. We reported the first example of a heteroatom-directed intramolecular hydroacylation. In this reaction, sulfide-containing substrates underwent hydroacylation with Wilkinson's catalyst to produce medium-ring sulfur heterocycles (eq 1). We observed that the success of the reaction depended on the not just the presence of the sulfur atom, but also its position relative to the aldehyde. While b-thioaldehydes underwent hydroacylation, the reaction failed for g-thioaldehyde substrates (Bendorf, et al., Tetrahedron Letters, 2002, 7031.).
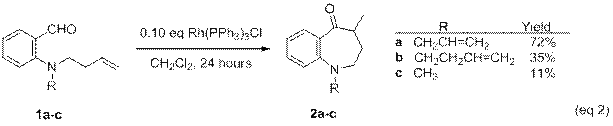
On-going work in our laboratory is aimed at adapting this heteroatom-directed hydroacylation strategy to the synthesis of medium-ring nitrogen heterocycles, compounds that display a broad range of biological activity. Initial work on this project examined the reaction of several 2-(3-butenylamino)benzaldehyde substrates with Wilkinson's catalyst, Rh(PPh3)3Cl (eq 2). Hydroacylation products were obtained in each case; however yields of product were influenced by the non-reacting amine substituent, R. Significantly higher yields of hydroacylation product were obtained for the allyl amine, 1a, suggesting that the allyl substituent also coordinates to rhodium. In all cases, the only significant product obtained (other than recovered starting material) was that due to hydroacylation of the butenyl alkene. Products due to decarbonylation or rhodium-mediated cleavage of the allyl group from the amine were not observed.
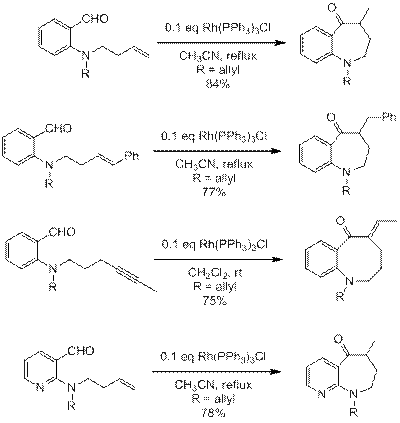
In the most recent grant period, our efforts focused on demonstrating the synthetic utility of this chemistry. A variety of substrates were prepared and subjected to the optimized hydroacylation conditions. Representative examples are shown in Figure 1. Mono- and di-substituted alkenes and alkynes readily undergo hydroacylation, however the reaction fails for tri-substituted alkenes. Benzazocines are also accessible by the hydroacylation of 4-alkynyl substituted amines, although the analogous alkenes fail to react. The presence of an aromatic amine functionality, such as a pyridine ring, does not interfere with the reaction.
Figure 1
Initial experiments with cationic rhodium catalysts, such as [Rh(dppe)]BF4, have also yielded medium-ring heterocycles. Allyl substituents, which do not undergo hydroacylation with Wilkinson's catalyst, readily cyclize with the more reactive cationic catalyst (eq 3). The analogous butenylamine substrate yields a mixture of products (eq 4). On-going work in our lab is directed at improving the selectivity and expanding the scope of this reaction.
Work on this project has been completed by undergraduate students at Lycoming College. Funds from this grant supported two students during the summer of 2011: Caitlin DeAngelo and Katherine Wellmon. Caitlin DeAngelo and Katherine Wellmon are both juniors and continue to work on this project. Kyle Ruhl and Kate Williamson worked on the hydroacylation project during the 2010-2011 academic year and were indirectly supported by this grant. Kyle and Kate both graduated in May 2011 with B.S. degrees in chemistry. Kyle is now enrolled in the PhD program in chemistry at Colorado State University. Kate is pursuing doctoral studies in chemistry at Oregon State University.