www.acsprf.org
Reports: DNI549557-DNI5: Transport Kinetics of Surfactant Molecules between Emulsion Particles Probed by Surface Specific Second-Harmonic Generation
Elsa Yan , Yale University
Introduction
Emulsions are widely used in industries and environmental
protection. The breaking and reformulation of emulsions, which occurs during
their use, lead to changes in their surface composition as well as physical and
chemical properties. Hence, a fundamental understanding of the transfer of
surfactant molecules between emulsion particles is required to optimize their
applications. However, such an understanding remains elusive due to the lack of
in situ and real-time surface-specific techniques. To tackle this problem, we
designed and synthesized surfactant probe molecules (Sp), referred to as
MG-butyl-1 and MG-octyl-1, which contain an n-butyl and n-octyl chain,
respectively, and a charged head group similar to malachite green (MG). MG is known to be effective in generating second harmonic generation (SHG)
signals when absorbed onto surfaces colloidal microparticles. Making use of the
coherent nature of SHG, we monitored the transfer of MG-octyl-1 and MG-butyl-1 between
oil-in-water emulsion particles of ~220 nm diameter. We found that MG-octyl-1 transfers ~700 times slower than MG-butyl-1, suggesting increases in
hydrophobic chain length decrease the transfer rate.
Experimental
Methods
Figure
1. The SHG kinetic measurement of surfactant molecules transferring between
emulsion particles: Schematic time-dependence of ISHG upon
addition of acceptor particles to donor particles at t = 0.
Due to the coherent nature of SHG, the SHG intensity (ISHG) depends quadratically on surface population of the adsorbed molecules on individual particles (N) but linearly on the particles density (ρ):
(1).
We measure the rate of surfactant probe molecules transport from donor to acceptor particles, where the acceptor particles (AP) are plain emulsion and donor particles (DP) are emulsion adsorbed with Sp that provide strong SHG signals. First, the SHG intensity is measured from the DP at a particle density, ρ. Then, an equal volume of AP at the same particle density is injected at t = 0. Although the overall particle density stays the same, the density of DP is halved. Hence, ISHG becomes ρN2/2. At t > 0, Sp transfer from the donor to AP leading to a decay of ISHG until a new equilibrium is established (t → ∞), where the Sp are equally distributed between the DP and AP. At equilibrium, the particle density becomes ρ and the surface population becomes N/2 such that ISHG becomes ρN2/4. Thus, the SHG intensity at time t < 0, t = 0 and t → ∞ are in the ratio of 1: 0.5: 0.25 (Fig. 1) and the decay of the SHG signal reveals the kinetics of surfactant transport between emulsion particles.
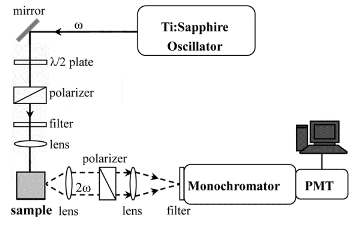
The setup of the SHG system is shown in Fig. 2.
Figure 2. The SHG
setup.
The oil/water emulsion was prepared by mixing 5 mM 1-dodecanol in n-tetradecane with 5mM sodium dodecylsulfate (SDS) in water at a volume ratio of 1:9 followed by ultrasonication for 6 minute.
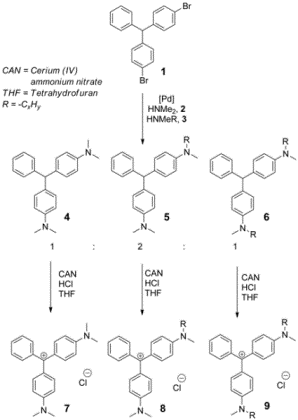
In order to perform the SHG experiments, we needed to synthesize
the Sp molecules. The surfactant probes MG-octyl-1 and
MG-butyl-1, were prepared by Buchwald-Hartwig coupling.
Figure
3. Synthesis of surfactant probe molecules Sp.
Results and Discussion
The pH of both DP and AP solutions was adjusted and maintained by a buffer (1 mM sodium phosphate, pH 6.2). Dynamic light scattering was used to measure the size of emulsion, yielding an average diameter of 226 ± 32 nm.
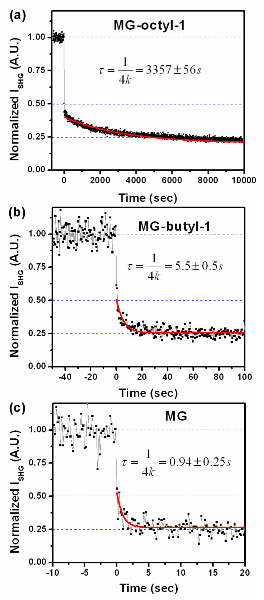
To obtain the kinetic data shown in Fig. 4, at t < 0, the emulsion was mixed with Sp to yield a final particle density at 1.3 × 1010 particles/cm3 and a final Sp concentration at 2.3 mM. At t = 0, an equal volume of plain emulsion at the same density was injected and the SHG intensity was monitored. Fig. 4 shows the time-dependence of the SHG intensity, which is in agreement with the theoretical predicted intensity at t < 0, t → ∞ in the ratio of 1:0.5:0.25. The normalized SHG intensity at t > 0 is fitted into the equation,
(2),
to
yield the transport rate constant, k, of (7.3 ± 0.2) × 10-5 s-1
for MG-octyl-1, (5.2 ± 1.6) × 10-2 s-1 for MG-butyl-1,
and 0.24 ± 0.03 s-1 for MG. A decrease of the alkyl length from
eight to four carbons leads to an increase of rate by ~700 times. Further
shortening the chain from butyl to methyl increases the rate by ~5 times. The
results suggest that the longer the alkyl chain the slower the transfer rate. Hence,
our data reveals that the hydrophobic interactions between the surfactant's
alkyl chains and the dispersed oil phase of the oil-in-water emulsion play an
important role in the rate-determining step of the transfer process.
Figure
4. The SHG kinetic data for (a) MG-octyl-1, (b) MG-butyl-1, and (c) MG. The
data are shown in dots and fitted by Eq. 2 (red curves).
The synthetic method described here can be used to make a
wide range of Sp for the SHG measurements. The method is versatile
because Sp can be synthesized by coupling a MG head group to one or two
alkyl chains via the Buchwald-Hartwig reaction using a variety of secondary amines. These amines can contain linear, branched or cyclic aliphatic or
aromatic hydrocarbon chains that are commercially available, or accessible by
reported synthetic procedures. Such a variety of Sp are expected to be
useful for studying the effect carbon chains on surfactant transfer between
colloidal emulsion particles, yielding practical information for developing new
surfactant molecules.
Conclusion and Future Directions
To summarize, we have designed and synthesized two Sp molecules, MG-octyl-l and MG-butyl-l containing an n-octyl and n-butyl chain, respectively. We have observed the transfer of MG-octyl-l or MG-butyl-l between oil-in-water emulsion colloidal particles in situ and in real time. Our studies support that a combination of SHG spectroscopy and molecular design and synthesis of Sp introduces an in situ and real-time technique to study kinetics of surfactant molecules transfer in colloidal emulsion systems.