www.acsprf.org
Reports: G647880-G6: Molecular Basis of Tetrahydrofuran-Induced Enclathration
Dilip Asthagiri , Johns Hopkins University
The goal of this project was to understand the role of tetrahydrofuran (THF) in promoting the enclathration of methane. A molecular-scale understanding of this process requires understanding how additives change the solubility of the solute of interest (here methane). In this concluding report, I summarize key achievements, ongoing challenges, and future path.
The excess free energy of a solute (methane) in solvent (water) determines its solubility, and is also of first interest in understanding hydrate formation. An added cosolute, for example THF, can change the free energy of the solute by influencing the properties of the solvent or by direct interactions with the solute. We have been developing theoretical approaches to dissect these distinct contributions to hydration.
Regularizing binding energy distributions to calculate free energies: We developed a new approach to calculate the hydration free energy, one that sidesteps the current dominant paradigm based on alchemically changing solutes. Our approach, a generalization of the quasichemical organization of the potential distribution theorem, rests on appreciating and exploiting the different energies with which a solute interacts with a solvent at different spatial scales. The approach leads to a transparent accounting of the hydration thermodynamics and is readily applicable to systems modeled by ab initio potentials or molecularly complex solutes such as THF, neopentane, or even macromolecules.
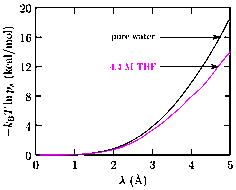
The high-energy tail of the distribution of solute-solvent interaction energies is poorly characterized for condensed systems (Fig. 1), but this tail region is of principal interest in determining the excess free energy of the solute. We introduce external fields centered on the solute to modulate the short-range repulsive interaction between the solute and solvent. This regularizes the binding energy distribution and makes it easy to calculate the free energy of the solute with the field. Together with the work done to apply the field in the presence and absence of the solute, we calculate the excess chemical potential of the solute.
We have applied this technique to study the hydration free energy of water using both classical (Fig. 2) and ab initio simulations (Fig. 3), and of hydrate promoters such as ethylene oxide (ETO), trimethylene oxide, and THF (Fig. 4).
Our studies on hydrate promoters suggest deficiencies in the forcefield for these molecules. For example, the hydration free energy of THF is somewhat more positive than the experimental value of about -2.5 kcal/mole. This likely explains why we barely see any changes in the solubility of methane in a 4.4 M solution of THF (data not shown). The presence of THF in pure water does increase the probability of forming voids in the medium (Fig. 5), a feature that suggests that solubility of methane should be enhanced in a THF-water mixture, as is also observed experimentally.
Coordination structure of water: We developed an approach to assess how various coordination states of a solute influences its free energy. We have used this to understand how various hydration states of a solute water influences its hydration thermodynamics. We had previously shown the importance water occupancy variation in an empty coordination sphere has for the coordination structure of ions. For water the surprising finding (J Chem Phys, 134, 124514, 2011) was that the free energy to form four water and one water clusters (in the presence of bulk medium) are nearly the same. Thus in the observed tetra-coordinate structure of the solute water, only one water molecule can be considered chemically bonded to the solute water. The remaining water molecules reflect the occupancy variation of the pure solvent.
External molecular fields for hydration: In the molecular aufbau approach we developed, we assess the local contribution in the presence of the external solvent medium. We tried investigating ways to describe the effect of the medium outside the coordination sphere by means of external fields. This approach failed, except for weakly hydrated species such as a methane or a solute water. In investigating this behavior for ions, we found that the bulk medium can stabilize configurations of the cluster that are usually not observed in the gas phase, while also simultaneously lowering the hydration free energy of the solute (J Chem Phys, 135, 054505, 2011). We expect this study to be relevant to experimental studies that translate thermochemistry of ion-water clusters to the thermodynamics of the hydrated ion and to evolving theoretical approaches that combine high-level calculations on clusters with coarse-grained models of the medium.
Understanding the role of THF in affecting water structure: To explore how THF enhances or diminishes solvent structure, we are exploring whether a suitably defined tetrahedral order parameter can provide insights into how local water structure in the presence of the additive.

Fig. 1: The problem of the high-energy tail and the regularization approach.

Fig. 2: Components of the free energy of a classical model of water using the
regularization procedure. Include the local chemical and packing contributions
(kT ln xs/ps) and outer contributions leads
to a consistent prediction of the free energy for all λ values. The large
λ values render the binding energy nearly Gaussian (Fig. 3 below). (J.
Chem. Phys. Under review, 2011) for further details.
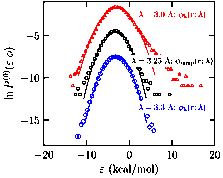
Fig. 3: The binding energy distribution of a water molecule with solvent water
in the presence of an external field. The configurations are obtained using ab initio molecular dynamics. Φ is
the field; notice that when the solvent water is pushed farther away (denoted
by a larger λ) the binding energy is better behaved and can be described
by a Gaussian.
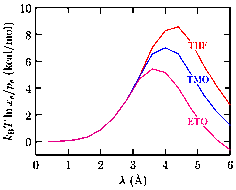
Fig. 4: The local chemical and packing contributions in the hydration of
various hydrate promoters. After including the long-range interaction
contributions, the hydration free energies of THF, TMO, and ETO are,
respectively, -1.0, -1.6, and -2.8 kcal/mole.
Fig. 5: Effect of THF in lowering the free energy for forming voids in water. The y-axis gives the free energy for opening a soft-cavity (J Chem Phys, under review 2011) in water.