www.acsprf.org
Reports: UNI350352-UNI3: Toward the Development of Iron-Based Olefin Metathesis Catalysts
Yann Schrodi, PhD , California State University (Northridge)
Prior to the start of this award, we developed a straight forward and atom economic method for the preparation of a new ruthenium phenylindenylidene complex 2. This method involved reacting RuCl2(p-cymene)(PCy3) with organic precursor 1a. The catalytic activity of complex 2 in the ring-closing metathesis (RCM) of diethyldiallyl malonate is very close to that of the related commercial 1st generation Hoveyda olefin metathesis catalyst under standard conditions. During the first year of this project, we further developed this method and attempted to use it to prepare indenylidene complexes of rhodium and iron.
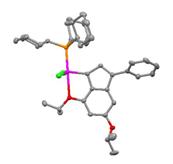
We became interested in exploring organic precursors 1b and 1c in place of 1a because 1b and 1c are very readily accessible and can be prepared without the use of n-butyllithium, contrary to the preparation of 1a. However, reactions of 1b and 1c with RuCl2(p-cymene)(PCy3) and RuCl2(PPh3)3 did not afford ruthenium indenylidene complexes. Organic precursor 1d was also synthesized and led to the formation of indenylidene complex 3, which possesses a pseudo trigonal pyramidal structure with both chloride ligands and the indenylidene carbon occupying the equatorial positions, as revealed by X-ray crystallography (Figure 1). This structure is similar to that of other related olefin metathesis catalysts, but is different than that of complex 2 according to NMR data. Nevertheless, the catalytic activities of 2 and 3 in the RCM of diethyldiallyl malonate under standard conditions are comparable.
Figure 1. X-ray structure of complex 3.
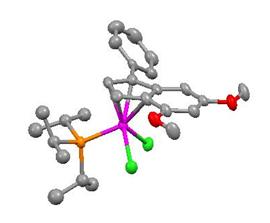
In an effort to determine whether these organic precursors can be used to prepare indenylidene complexes of other metals, organic precursor 1a was reacted with the [RhCl(PiPr3)2]2 dimer. This reaction afforded a rhodium vinylidene complex 4, which gave a rhodium indenyl complex 5 upon treatment with an excess of HCl. Both of these complexes were characterized by X-ray crystallography. The formation of the rhodium indenyl (Figure 2) suggests that the vinylidene complex was first converted to a rhodium indenylidene intermediate, which was protonated to the indenyl compound 5.
Figure 2. X-ray structure of complex 5.
Iron complexes P2FeCl2 and (P-P)FeCl2, where P is di-t-butylmethylphosphine and P-P is 1,2-bis(dicyclohexylphosphine), were prepared and reacted with a derivative of organic precursor 1a labeled with 13C atoms in both acetylenic postitions. 1H and 13C NMR spectroscopy revealed that no reaction occurred in both cases even after refluxing in tetrahydrofuran for 3 days. Our current efforts are focused on exploring the reaction between organic precursors 1a and 1d with other iron-based complexes.
Two undergraduate (Shuai Wang and Daniel Tolentino) and three graduate students (Wing-Sy Wong DeRieux, Matthew Ryan, and Daniel Tabari) actively participated in this project. Shuai Wang is currently applying to Ph.D. programs in chemistry, while Daniel Tolentino and Wing-Sy Wong DeRieux are seriously considering doing so in the future. Matthew Ryan's goal is to find a research position in the private sector or teach chemistry in high school or college. Daniel Tabari aspires to become a physician and is currently preparing to interview with medical schools.