www.acsprf.org
Reports: UNI749338-UNI7: A Systematic Approach Towards the Synthesis of Metal Coordination Cross-Linked Polymer Networks
Alexander D. Schwab , Appalachian State University
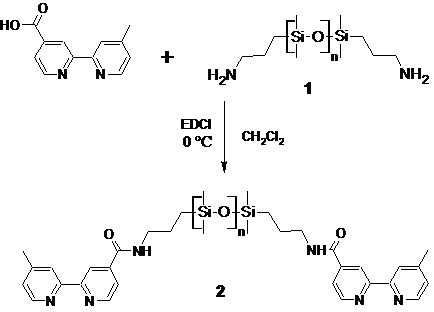
The overall goal of this project is to investigate whether an elastomeric (rubber) material can be made utilizing metal coordination complexes as cross-links rather than covalent bonds. To achieve this goal, polymer molecules terminated with metal binding ligands have been synthesized. The originally proposed synthetic scheme for attaching metal-binding bipyridine groups (bpy) to the ends of amine terminated poly(dimethylsiloxane) (PDMS) was abandoned in favor of a faster, safer, cleaner, and simpler N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDCI) coupling scheme. Undergraduate students Meredith Hyatt and M. Lee Pippin implemented the EDCI scheme below using 2500 g/mol amine terminated PDMS (1).
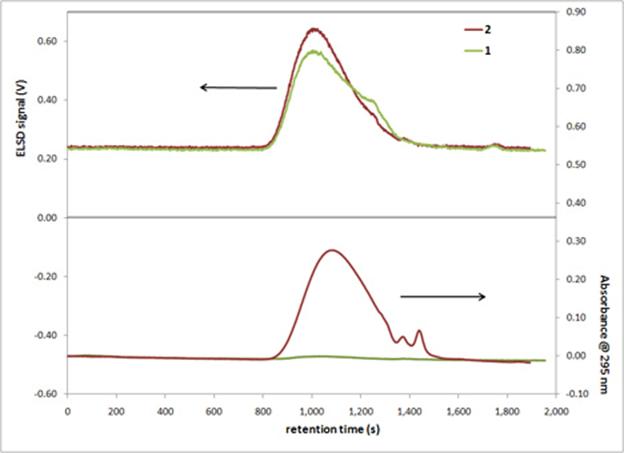
In the Fall of 2010, Kurtis Price developed a gel permeation chromatography (GPC) method for characterizing amine (1) and bipyridine (2) terminated PDMS products as part of his senior research project (CHE 4400). Meredith and Lee used this method to characterize their PDMS products. An example chromatogram is shown in Figure 1. The evaporative light scattering detector (ELSD) is sensitive to non-volatile analytes (i.e. anything other than the mobile phase), and the UV-vis detector set to 295 nm is sensitive to the bipyridine moiety. The peak in UV-vis response for 2 at nearly the same retention time as the ELSD peak for 1 indicates that bipyridine was successfully attached to 1 without any undesirable chain coupling. The 1H NMR spectrum of 2 indicates the complete conversion of the amine group to an amide group further confirming the successful conversion of 1 into 2.
Figure 1: GPC chromatograms with ELSD response (top) and UV-vis response (bottom) for samples of 1 and 2 injected at time 0. The mobile phase was 100:1 chloroform:n-butylamine (v:v) with a flow rate of 1 mL/min through 2 x 25.0 cm Jordi Labs X-stream H2O 500Å columns.
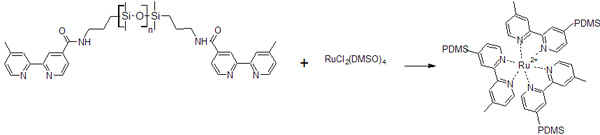
Convinced that we have synthesized the desired bpy terminated PDMS, 2, Meredith and Lee prepared a series of methanol solutions containing 2 with a soluble source of Ru2+: RuCl2(DMSO)4. The bpy ligands should displace the DMSO ligands on Ru2+ leading to the formation of a tri-functional cross-link as shown in the following scheme (counterions and DMSO omitted in product). UV-vis spectra from one of the methanol solutions over time are shown in Figure 2.
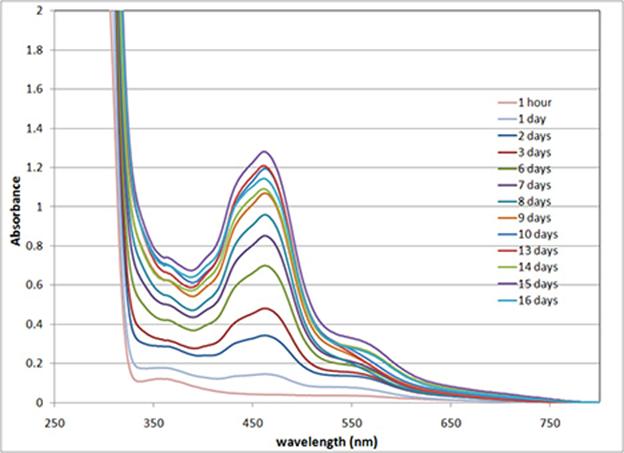
Figure 2: UV-vis absorbance spectra of diluted samples from a methanol solution that is 13 mM in 2 and 8.7 mM in RuCl2(DMSO)4 (i.e. 3:1 bipyridine:Ru2+)
In general, a peak at 455 nm, which can be assigned to the formation of Ru(bpy)32+ complexes, grows over time. Direct interpretation of the spectra is complicated by the buildup of an insoluble fraction of material on the walls and bottom of the glass container over time. The spectra above are for samples of the freshly agitated methanol solution and do not reflect complex formation in the insoluble fraction. This insoluble coating is likely formed because methanol is a poor solvent for PDMS and, upon cross-linking, the material should become insoluble. When the methanol solution is decanted, the remaining coating on the walls of the container is a deep red color, indicating that coordination cross-linking may be one of the driving forces for this material coming out of solution. The deep red material has a viscosity much higher than that of the parent amine terminated polymer (1) as expected, though it isn't particularly rubbery.
Our ongoing work on this project is proceeding on a few different tracks. First, we are attempting to use our current synthetic method to create bipyridine terminated poly(ethylene glycol) (1,500 g mol-1) and PDMS (27,000 g mol-1) molecules. To date, 1H NMR spectra of both products indicate successful conversion of amine groups to amide groups. Initial GPC characterization of the PEG product indicates that the EDCI coupling might have caused a significant increase in the polymer molecular weight. Further work is needed to rule out potential polymer/column interactions. The 500Å GPC columns currently employed are not suitable for characterization of the higher molecular weight PDMS. A new set of mixed bed GPC columns has been purchased and will soon be tested and used.
Second, we're working with a simple synthetic method that converts the amine groups in 1 to pyridine-imine ligands as described in the original grant proposal. 1H NMR spectra indicate complete conversion of the amine end groups to pyridine imine. GPC results indicate that our pyridine-imine end groups might decompose on the column or that there is a signicant source of contamination in our product. Regardless, we've performed experiments, similar to those illustrated in Figure 2, with the pyridine-imine terminated PDMS molecules and have found spectroscopic evidence for coordination cross-linking in those systems as well.
Third, we are attempting to optimize the solvent and stoichiometry for the metal coordination cross-linking process in order to increase the rate of complex formation. To date, methanol appears to be the best solvent choice to maximize the amount of final complex formation as well as the rate of complex formation. Additionally, we're experimenting with the use of AgPF6 to remove the Cl- from Ru2+. The more weakly coordinating PF6- anions might afford better complex formation.
Finally, we are currently investigating potential collaborations to better characterize our cross-linked products. Techniques we'd like to employ include differential scanning calorimetry to observe changes in glass transition temperature, and oscillating shear rheometry to characterize the viscosity increase upon cross-linking.