www.acsprf.org
Reports: AC246636-AC2: Quantum Mechanical Calculation of Hydrogen Isotope Exchange Thermodynamics and Kinetics on Petroleum Model Compounds
James D. Kubicki , Pennsylvania State University
Molecular H isotopes can be used to interpret the source(s) of petroleums accumulated in reservoirs. Knowledge of the thermodynamics and kinetics of H/D exchange at various types of C sites will facilitate such applications by enabling us to evaluate the imprint of isotopic exchange on molecular signatures.
The fourth year of research into the thermodynamics and kinetics of H-D isotopic exchange on kerogen-related molecules was conducted by performing calculations on H abstraction and H-D exchange reactions with hydroxyl groups representative of those on mineral surfaces (e.g., clays). As kerogen interacts with the minerals of the host rock or as petroleum migrates through sedimentary rock, H-D exchange may occur. This research will be used to help interpret observed H-isotope fractionation factors in ancient organic matter from sedimentary rocks. Theoretical values are needed because molecular-specific isotopic analyses have recently been made possible, but experimental values are difficult to obtain especially for specific sites within compounds. Observed fractionation trends will require knowledge of both the equilibrium fractionation factors and exchange rates as a function of temperature. Knowledge of isotopic H-D fractionation factors between kerogen and petroleum components and mineral surfaces is limited.
The B3LYP/6-311++G(d,p) method was used to calculate vibrational frequencies of the organic compound 2-methyl butane (Fig. 1) and molecular clusters representive of mineral surface groups. The calculated frequencies were used to estimate ln(b) values for alkane compounds. The α values were then calculated by the difference between the alkane ln(b) values. This method of calculating ln(b) values has been previously tested by comparing the model ln(β) value against the known value for water vapor.
The reaction half-lives were then found from t1/2 = ln(2)/k and are listed in Table 1. Without H2O, the half-lives indicate that isotopic exchange rates will be so slow via H abstraction that this reaction can be ignored within geologic time. The half-lives are all significantly longer that measured for stereochemical inversion in pristane incubated on shale with elemental sulfur by Abbott et al. indicating the other reaction paths are necessary to induce significant H-D exchange on alkanes. We reiterate that these activation barriers are reasonably accurate because the experimental value for methane is 438 kJ/mol. Note that increasing the (q*/qreact) ratio to 10 would decrease these half-lives by one order of magnitude.
Without H2O, the half-lives indicate that isotopic exchange rates will be so slow via H abstraction that this reaction can be ignored within geologic time. The half-lives are all significantly longer that measured for stereochemical inversion in pristane incubated on shale with elemental sulfur by Abbott et al. (Abbott et al., 1985) indicating the other reaction paths are necessary to induce significant H-D exchange on alkanes. We reiterate that these activation barriers are reasonably accurate because the experimental value for methane is +438 kJ/mol. Note that increasing the (q*/qreact) ratio to 10 would decrease these half-lives by one order of magnitude.
The most important conclusion of this study of organic-mineral H-D isotope exchange is seen in Table 2. The calculated activation energies are in the range of 140 to 300 kJ/mol. Compared to calculated H-D exchange activation energies for 2-methyl butane and H2O (in the range of 200 to 300 kJ/mol), these can be dramatically lower for all C-H sites when interacting with surface aluminol groups. Consequently, we conclude that H isotope exchange can occur at significantly lower temperatures and with faster rates when kerogen and petroleum interact with aluminosilicate mineral surfaces. Because the surface hydroxyl H isotope signature is determined by interactions with pore waters, this ends up being a way to catalyze H isotope exchange between water and organic matter at depth either in the source rock or during migration of fluids.
Table 1 Estimated half-lives (t1/2) in years for MP2/ and B3LYP/6-311++G(d,p) H abstraction reactions on 2-methyl butane with and without H2O present.
Temperature a-primary b-primary Secondary Tertiary____
w/out H2O
B3LYP/6-311++G(d,p)
25°C 0.10E+51 0.33E+51 0.92E+49 0.76E+44
100°C 0.56E+36 0.15E+37 0.81E+35 0.70E+31
150°C 0.11E+30 0.26E+30 0.20E+29 0.52E+25
200°C 0.55E+24 0.12E+25 0.12E+24 0.76E+20
250°C 0.29E+20 0.57E+20 0.72E+19 0.91E+16
MP2/6-311++G(d,p)
25°C 0.35E+41 0.17E+42 0.83E+38 0.15E+34
100°C 0.15E+29 0.55E+29 0.12E+27 0.20E+23
150°C 0.23E+23 0.73E+23 0.33E+21 0.15E+18
200°C 0.60E+18 0.16E+19 0.13E+17 0.14E+14
250°C 0.11E+15 0.28E+15 0.37E+13 0.74E+10
with H2O
B3LYP/6-311++G(d,p)
25°C 0.81E+14 0.22E+10 0.21E+13 0.66E+09
100°C 0.79E+07 0.18E+04 0.44E+06 0.69E+03
150°C 0.40E+04 0.25E+01 0.31E+03 0.10E+01
200°C 0.99E+01 0.13E-01 0.10E+01 0.62E-02
250°C 0.76E-01 0.19E-03 0.96E-02 0.96E-04
MP2/6-311++G(d,p)
25°C 0.40E+25 0.79E+24 0.11E+21 0.20E+16
100°C 0.28E+16 0.76E+15 0.63E+12 0.10E+09
150°C 0.14E+12 0.44E+11 0.84E+08 0.39E+05
200°C 0.54E+08 0.19E+08 0.72E+05 0.75E+02
250°C 0.94E+05 0.38E+05 0.24E+03 0.48E+00
Table 2: Calculated activation barrier energies (in kJ/mol) for H abstraction from each 2-methyl butane H site with each type of surface hydroxyl (see Fig. 2). The activation barrier energies are calculated from the energy-minimized structure of 2-methyl butane and the O of the surface. The bonds were brought together in 0.2 Å increments up to a distance of 1.05 Å for the O-H bond. Two sites for the Si surface were examined: SiO- and SiOH. _______________________________________________________________________
Molecule Site Al-OH Al-H2O SiO- SiOH
2-Methyl Butane Alpha 148 184 185 287
Beta 140 180 193 282
Secondary 155 160 195 295
Tertiary 168 175 188 290
|
|
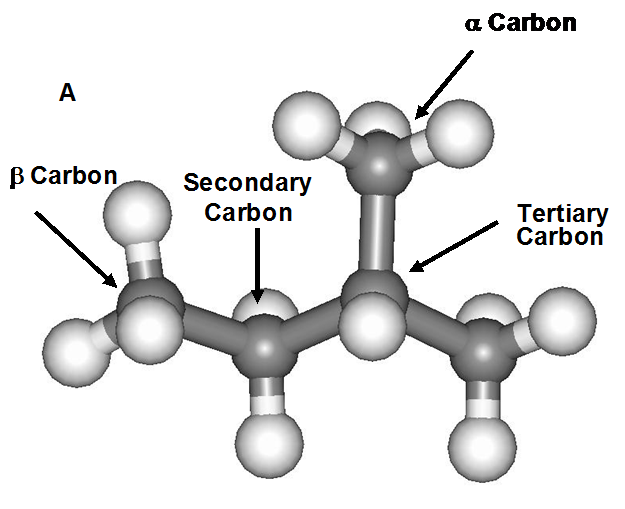
Figure 1: The molecule structures that were modeled a) 2-methyl butane and b) 2,6-octane. The four separate H positions are labeled in each molecule tertiary, secondary and two primary H atoms (one a and one b to the tertiary C atom).
|
|
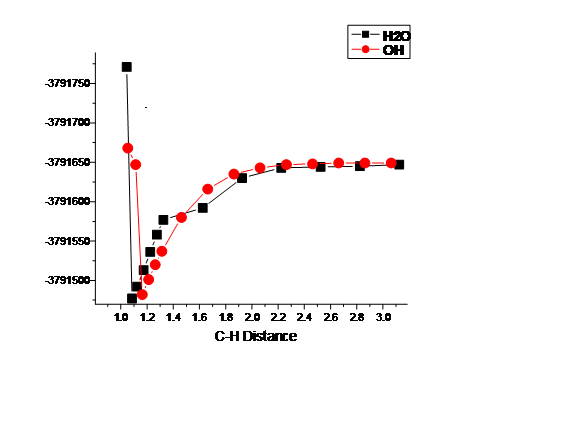
Figure 2: H abstraction between the reaction pathway between a model Al2(H2O)4(OH)6 surface and the secondary carbon site on 2-methyl butane. A H2O site on the Al surface, deprotonates after 2-methyl butane deprotonates, thus 2-methyl butane accepts the H from the Al-OH2 site.
References
Abbott G. D., Lewis C. A., and Maxwell J. R. (1985) Laboratory models for aromatization and isomerization of hydrocarbons in sedimentary basins. Nature 318, 651-653.
