www.acsprf.org
Reports: B348504-B3: Synthesis, Characterization, and Reactivity of Platinum/Ruthenium Heterometallic Complexes
Nancy Carter Dopke , Alma College
Progress has been achieved in multiple distinct arms of the overall project studying the influence of structural changes in platinum-ruthenium complexes on the reactivity with alcohols in atmospheric oxygen. Three undergraduate students did research during academic terms and one worked during the summer on the synthesis and reactivity of heterometallic complexes.
Work concluded on the optimization of the synthesis of monomer platinum phosphine complexes by microwave synthesis. The use of a microwave system decreased the time of the reaction for the synthesis of dppePtCl2 from 8 h to 1 h and increased the percent yield from 47.1% as the best achieved by conventional synthesis to 71.6% as the best achieved by microwave synthesis. With minor modifications, the complexes dppmPtCl2, dpppPtCl2 and cis-(Ph3P)2PtCl2 can also be synthesized in good yields and with reaction times of 1 h or less.
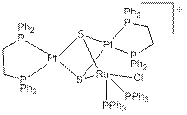
Previous work on the project established the synthetic procedure and full characterization of [(dppePt)2(m3-S)2Ru(PPh3)2Cl]Cl (dppe = 1,2-bis(diphenylphosphino)ethane). One student's work is focused on the reactivity and transformations of this complex. The complex is synthesized through a stepwise procedure. While the synthesis of the monomer platinum phosphine has been optimized to yields as high as 71.6%, the next step in the synthesis, the synthesis of the dimer species (dppe)2Pt2(m-S)2, continues to be a challenge with impure products and low yields. The complex (dppe)2Pt2(m-S)2 is produced as an impure sample that has then been purified by reaction with Ru(PPh3)3Cl2 to produce the trimer species [(dppePt)2(m3-S)2Ru(PPh3)2Cl]Cl. The reactivity of this complex was investigated by measuring its ability to oxidize neopentyl alcohol in atmospheric oxygen. The complex has a low turnover under atmospheric oxygen at 60°C. The platinum starting materials, dppePtCl2 and (dppe)2Pt2(m-S)2, have no ability to oxidize neopentyl alcohol under the same conditions and the alcohol is not oxidized in the absence of a metal containing species. Attempts are underway to remove one of the sulfur bridges which should result in a greater interaction between the metal atoms. The established structure of the trimer species is shown below.
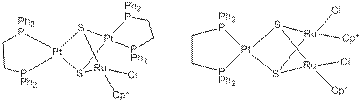
The reactivity of the ruthenium-rich complex, (dppe)Pt(m3-S)2(RuCp*Cl)2,and the platinum-rich complex, (dppePt)2(m3-S)2(RuCp*Cl), have been investigated by measuring their ability to oxidize phenyl ethanol in atmospheric oxygen. The ruthenium-rich complex is synthesized through a methodical stepwise procedure through a species with a Ru2(m2-S)2 core. The synthesis of the platinum-rich complex is non-methodical with the trimer being made from a Ru4 cube complex and monomer Pt complex. The platinum-rich complex with a Pt2Ru bridged core has a greater reactivity with the alcohol, though the product formed after 72 hours is still under the stoichiometric amount under atmospheric oxygen as determined through the use of an internal standard and 1H NMR. Issues with the loss of solvent during the 72 hour reaction time has been solved by the use of a single piece glass apparatus. Continued reactivity studies and further characterization is underway. The proposed structures of the two complexes are shown below.
Funds from the grant made it possible for an undergraduate student to contribute to the project over the summer. An undergraduate researcher presented a poster titled Comparison of Pt-rich and Ru-rich heterometallic complexes containing the ligands dppe and Cp* at the March 2011 National American Chemical Society meeting in Anaheim. Attendance at the meeting allowed the student to explore the vast topics of research in chemistry.