www.acsprf.org
Reports: UNI450467-UNI4: Photophysical Characterization of Free-base Bis(arylethynyl)porphyrin Diacids and Aggregates
Kathryn E. Splan , Macalester College
The narrow absorption profiles exhibited by porphyrins limit their use in many photophysical applications. Arylethynylporphyrins that feature expanded conjugation of the porphyrin macrocycle hold great potential as chromophores that exhibit enhanced light-harvesting properties in the red region of the spectrum. However, to date, use of arylethynylporphyrins in optical applications has centered primarily on metalloporphyrin derivatives. Our project extends aspects of free-base porphyrin chemistry to include arylethynyl porphyrin derivatives.
Results from Year 1.
 The
first year of the funding period has focused on the fundamental
characterization of free-base arylethynyl photophysics in both the neutral and
diacid form. To delineate the impact of the ethynyl linkers on free-base
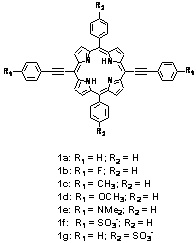
arylethynyl porphyrin photophysics, we completed the synthesis of arylethynyl porphyrins
1a-e and the trans-disubstituted H2TPP derivatives
lacking arylethynyl functionalities and conducted a comprehensive photophysical
study of the compounds. The majority of the synthetic efforts were done by
Peter Goldberg (Chemistry, '11) during the summers of 2009 and 2010, while
supported by Macalester College via internal funding. Peter then spent the Fall
of 2010 compiling electronic absorption and emission peak maxima, extinction
coefficients, Stokes shifts, and fluorescence quantum yields for the compounds
in both their neutral and diacid forms. Also during the funding period, we
initiated a collaboration with Prof. David Blank of the University of Minnesota
to allow for measurement of fluorescence lifetime data.
The
first year of the funding period has focused on the fundamental
characterization of free-base arylethynyl photophysics in both the neutral and
diacid form. To delineate the impact of the ethynyl linkers on free-base
arylethynyl porphyrin photophysics, we completed the synthesis of arylethynyl porphyrins
1a-e and the trans-disubstituted H2TPP derivatives
lacking arylethynyl functionalities and conducted a comprehensive photophysical
study of the compounds. The majority of the synthetic efforts were done by
Peter Goldberg (Chemistry, '11) during the summers of 2009 and 2010, while
supported by Macalester College via internal funding. Peter then spent the Fall
of 2010 compiling electronic absorption and emission peak maxima, extinction
coefficients, Stokes shifts, and fluorescence quantum yields for the compounds
in both their neutral and diacid forms. Also during the funding period, we
initiated a collaboration with Prof. David Blank of the University of Minnesota
to allow for measurement of fluorescence lifetime data.
Our results show that enhanced substituent effects on porphyrin absorption spectra are observed in the arylethynyl porphyrins relative to the H2TPP derivatives. Upon protonation, both series of porphyrins exhibit substantially red-shifted absorption and emission spectra and enhanced oscillator strengths, with the magnitude of the spectral shifts being more substantial in the presence of the ethynyl functionalities. Protonation of both series of porphyrins also results in reduced fluorescence lifetimes and enhanced nonradiative decay rates, and the impact of protonation on these parameters is attenuated in the presence of the arylethynyl functionalities. This study comprises the first photophysical investigation of free-base arylethynyl porphyrins and was recently published in The Journal of Physical Chemistry A.
Central to the success of this project and others ongoing in the Splan lab was the acquisition of an Agilent 8453 UV-Visible spectrophotometer, procured with PRF funds along with a Macalester College match. The instrument was ordered and installed during the summer of 2010 upon receipt of the award and has constituted a much needed addition to the laboratory infrastructure.
Future directions.
In the next year of the grant, we will extend our photophysical characterization to include tetraethynyl-substituted free-base porphyrins. Owing to the lower degree of structural distortion upon protonation relative to H2TPP, we anticipate that the impact of protonation upon photophysics will be further attenuated relative to the bis-substituted porphyrins. Initial efforts to synthesize 5,10,15,20-tetra(phenylethynyl)porphyrin (H4TPEP) have proved unsuccessful owing to the presence of small impurities that cannot be removed via column chromatography. During the summer of 2011, we will work to synthesize derivatives that bear additional solubilizing functional groups which will hopefully facilitate the purification process. Collaboration will be sought to measure triplet yields to substantiate the contribution of both intersystem crossing and internal conversion pathways to total nonradiative decay of the porphyrins.
Also in the upcoming year, we will synthesize and characterize porphyrins 1f and 1g. The sulfonate functionalities in 1f and 1g were chosen to impart water solubility on the porphyrins, rendering them amenable for aggregation studies. The formation of J-aggregates of 1f and 1g under acidic conditions will be studied via electronic absorption spectroscopy and the size and morphology of the aggregates will be assessed via atomic force microscopy. Owing to the variation in distance between contact points in J-aggregates formed from 1f and 1g, we predict that the aggregates will have different spectroscopic and morphological properties, demonstrating that the nature of porphyrin aggregate structures can be controlled synthetically.
Impact on Faculty and Students
Support from the Petroleum Research Fund has had a great impact on the success of the PI's career. The grant has provided support for summer salary and travel to conferences, supplies for the project, and funding for instrumentation that would not have been available through other sources. Support for the project has also allowed Peter Goldberg (supported internally by Macalester prior to PRF funding) to continue his research and bring the project to the point of publication. Peter presented his results at the National American Chemical Society meeting in March 2011, and he is now enrolled as a first year graduate student at the University of Michigan Department of Chemistry. Owing to the PI's pre-tenure sabbatical leave, no students were supported by PRF during the summer of 2011. However, PRF funds will be used to support two students during the summer of 2012 for the projects described above.
