www.acsprf.org
Reports: DNI1048810-DNI10: New Mechanisms of Hydrogen Storage from Chemical Frustration
Peihong Zhang, PhD , State University of New York at Buffalo
Thermodynamically, an ideal material for hydrogen storage must lie near the hydrogenation/dehydrogenation phase boundary. This stringent requirement is unlikely to be satisfied with materials involving only conventional interaction mechanisms (e.g., covalent, ionic, or van der Waals), and there is increasing interest in exploring unconventional and intermediate bonding mechanisms in the search for hydrogen storage materials.
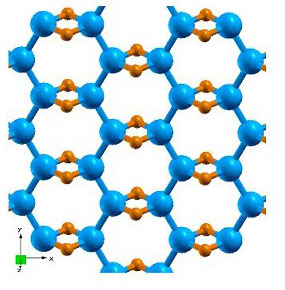 |
| Fig. 1. Structure of of the proposal single-layer B2H2. |
Recently, we proposed that the three-center two-electron (3c2e) boron-hydrogen bonds found in many boranes may be able to stabilize the planar boron structure to form layered solid B2H2 as shown in Fig. 1. This structure is isoelectronic to MgB2 and can be doped with electrons. We have also studied the effects of charging on the dynamical properties of B2H2 and found that some hydrogen related phonon modes are softened significantly, whereas boron modes are either hardened or not strongly affected. These findings suggests a charging-assisted hydrogen release mechanism.
In this period, we extend our work to address the hydrogen release dynamics and kinetics by carrying out ab initio molecular dynamics (MD) simulations and nudged elastic band (NEB) calculations. We have investigated both neutral and electron doped systems and found that charging the B2H2 structurewith electrons significantly lowers the dehydrogenation kinetic energy barrier and facilitates the release and possible formation of hydrogen molecules. The underlying boron backbone structure, in contrast, remains intact.
The electron-deficient hexagonal boron layer is stabilized through the formation of 3c2e with hydrogen. Therefore, when the boron layer acquires extra electrons (through doping or charging), it becomes less electron deficient. This in turn weakens the bridge hydrogen-boron bonds. Consequently, as the boron layer gets enough electrons to quench its electron deficiency, the hydrogen will no longer bound to the boron layer and gets released.
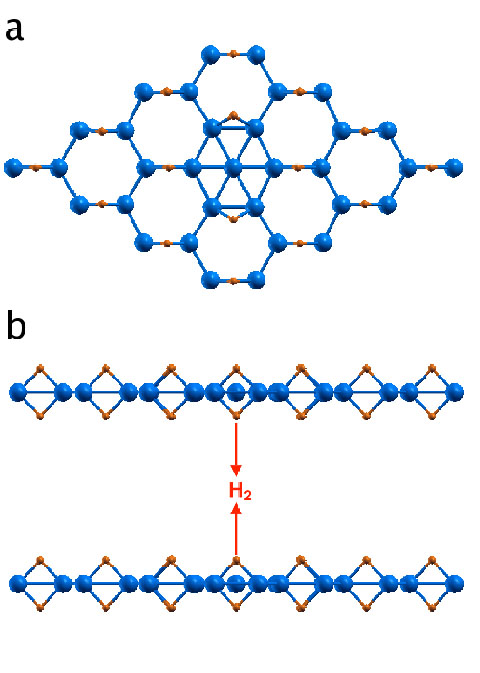
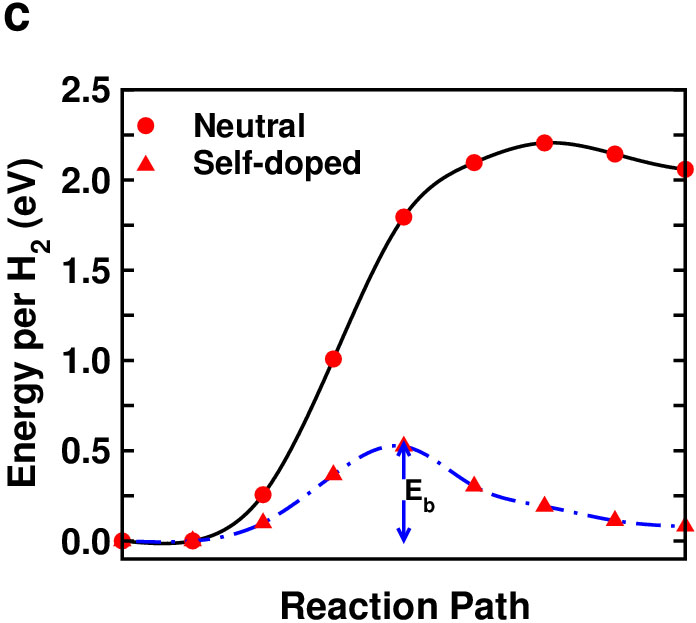
We first consider a scenario in which hydrogen atoms are released from two adjacent self-doped B2H2 layers and diffuse to merge together to form H2 as shown in Fig. 2 (a), (b). The calculated kinetic energy barrier for both a neutral nd a self-doped systems is shown in Fig. 2(c). It is clear that releasing hydrogen from the neutral B2H2 system would be very difficult. However, with the presence of a self-doped boron, the kinetic energy barrier is significantly reduced. The additional boron donates electrons to the B2H2 sheet, which makes the system less electron deficient and weakens the bridge B-H bonds, thereby facilitating the hydrogen release. Therefore, one may use charging (in this case, through self-doping) to tune the strength of the B-H bridge bonds and the dehydrogenation kinetic energy barrier.
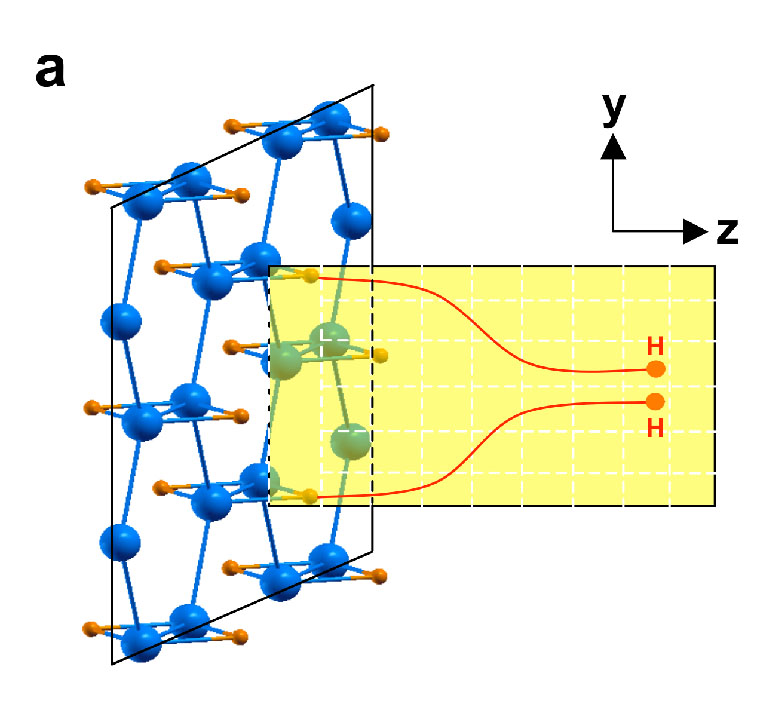
The second scenario that we consider involves the release of hydrogen and formation of H2 from a single B2H2 layer as shown in Fig. 3 (a). We introduce one additional electron per unit cell so that the system is isoelectronic to AlB2. We again observed significant lowering (by about 1.5 eV) of the dehydrogenation kinetic energy barrier (Fig. 3 (b)) when additional charges (electrons) are introduced into the system.
 |
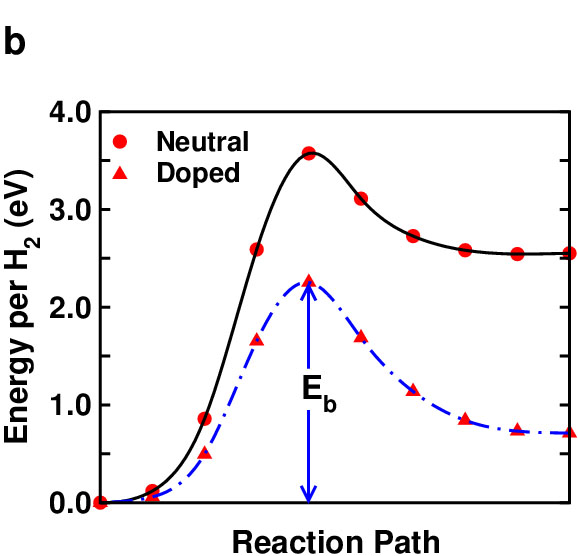 |
| Fig. 3. (a) A possible hydrogen release pathway from a single layer B2H2 and (b) kinetic energy barrier (Eb) as a function of a reaction path for such a process. | |
It is also possible that the two charge-doping mechanisms (i.e., boron self-doping and alkali metal intercalation) may be combined to achieve optimal results. While boron self-doping may serve as a convenient means to tune the strength of the B-H bonds, alkali atom intercalation may be introduced at the time of dehydrogenation. This brings an interesting possibility of utilizing the electrochemical reactions to facilitate the dehydrogenation process. It may also be interesting to investigate the possibility of combining the Kubas interactionmechanism with the B-H 3c2e bonding mechanism to fine tune the dehydrogenation kinetics and thermodynamics of boron hydride solids.
To further unravel the charging effects on the thermodynamics of the layered B2H2 structure and its dehydrogenation kinetics, we have performed an extensive Car-Parrinello molecular dynamics simulations for both neutral and charged B22 systems. We have performed a long-time simulation of more than 10 ps with a time step of 0.025 fs using 400 000 MD steps.
Our results show that the neutral B2H2 structure is sufficiently stable at least up to 500 K and that the strength of the B-H bridge bonds are indeed intermediate. However, we do not observe the release of hydrogen atoms or the formation of hydrogen molecules at the simulation temperatures.
After introduce extra changes to the system, the B-H bonds weaken significantly and release of hydrogen molecules are observed during the MD simulation, as shown in Fig. 4.
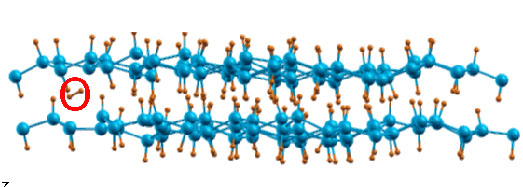 |
| Fig. 4. A molecular-dynamics simulation snapshot
of a charged B2H2 structure, which exhibits considerable
rearrangements of the H-B bridge bonds and a formation of an H |
In summary, we investgated the hydrogen release mechanism in a layered B22 system. The additional charges introduced into the B2H2 system reduce the electron deficiency of boron network and thereby modulate the strength of the B-H bridge bonds and dehydrogenation kinetic barriers. As a result, we find a significant decrease in the energy barrier required for hydrogen release upon charge doping. Our MD results show a substantial weakening and breaking of the B-H bonds with charging, which suggests a charging-assisted dehydrogenation mechanism. The boron framework remains stable for both the neutral and charged systems at least up to 500 K.