www.acsprf.org
Reports: DNI749220-DNI7: Templating Synthesis of Polymeric Nanocages via Interfacial Polymerization of Monomer-Functionalized Surfactant Monolayer Absorbed on Crystallized Microemulsion Nanodroplets
Chong Cheng, PhD , State University of New York at Buffalo
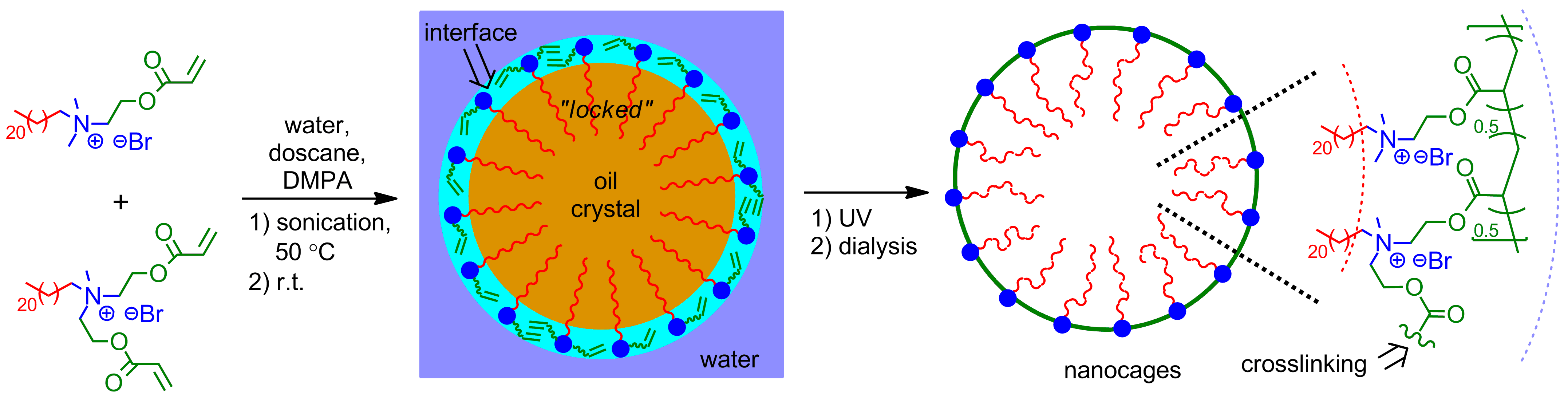
Novel nanocages with well-controlled monolayer-thick shell were successfully prepared by interfacial polymerization of surfactant monolayers adsorbed by crystallized nanodroplets (Scheme 1). Quaternary ammonium-based compounds having a long n-docosyl tail and with one and two acryoyl groups were used as monomer and crosslinker surfactants, respectively. 2,2-Dimethoxy-2-phenylacetophenone (DMPA) was selected as photoinitiator. A transparent reaction system (96.3 wt% of water, 2.0 wt% of docosane, [monomer surfactant]0:[crosslinker surfactant]0:[DMPA]0 = 18:14:1) was obtained after 30 min of ultrasonication of the reaction mixture at 50 °C. Then it was cooled down slowly to room temperature, and the occurrence of crystallization of the dispersed oil phase during the cooling was verified by DSC analysis. Interfacial polymerization of the surfactants was induced by UV irradiation for 30 min. Complete conversion of the acryoyl groups of the surfactants was verified by 1H NMR and FT-IR analysis.
Scheme 1. Template synthesis of nanocages with monolayer-thick shell via crystallized nanodroplets
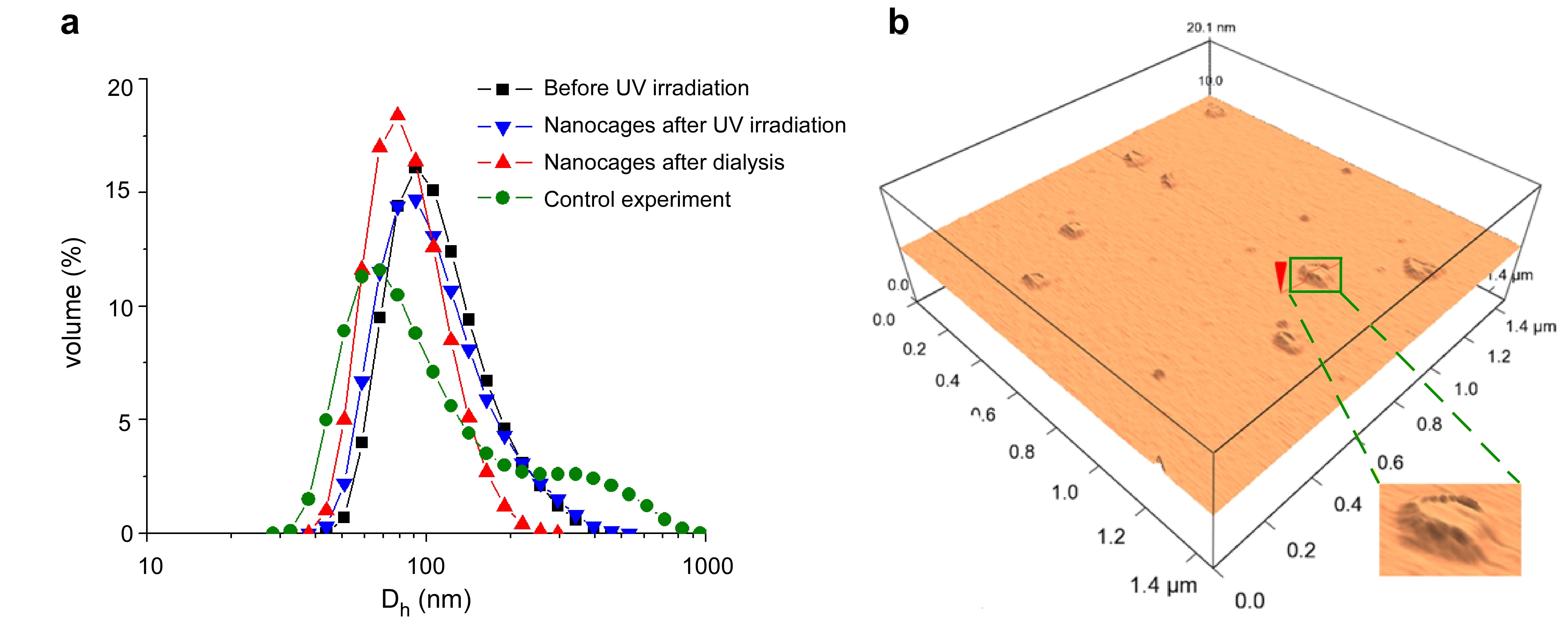
As monitored by DLS, the crystallized nanodroplets had a volume-average hydrodynamic diameter (Dh,v) of 118.1 nm before UV irradiation; the resulting nanodroplets covered by nanocages had a Dh,v of 113.6 nm after UV irradiation (Figure 1a). Their close agreement demonstrated the high template stability during the synthesis. After dialysis against THF, the nanocages showed a Dh,v of 88.7 nm due to the removal of docosane. Zeta-potential measurements showed positive surface charges of the dispersed phase throughout the process (before UV irradiation: 88 ± 3 mV; after UV irradiation: 75 ± 3 mV; after dialysis: 71 ± 5 mV). To identify the merit of template synthesis via crystallized nanodroplets, a control experiment was conducted for comparison. As a result, the interfacial polymerization at 50 °C with liquid nanodroplets as synthetic templates gave ill-defined product with a broad bimodal size distribution as revealed by DLS, indicating the occurrence of template destabilization during the polymerization process. The comparison illustrated that the stabilization of the synthetic templates via crystallization was critical for achieving precise template control in synthesis. The nanostructure of nanocages after dialysis was visualized by TEM and tapping-mode AFM. TEM imaging of nanocages on carbon film showed their capsule-like structures with sizes around 100 nm. As measured by AFM on mica, nanocages exhibited highly collapsed capsule-like morphology with surface diameters also around 100 nm (Figure 1b). Deduced from the surface height of ~0.8 nm of the central region of nanocages that corresponded to twice the thickness of the shell, the monolayer-thick shell of the nanocages had a thickness of only ~0.4 nm. With such a shell thickness, an average surface area of ~ 2 nm2 per polymerized surfactant molecule can be estimated based on the assumption of density of ~1g/cm3 of the shell on surface.
Figure 1. (a) DLS results for the synthesis of nanocages, (b) 3-D AFM height image of nanocages after dialysis.
In summary, a new and facile approach for the synthesis of novel nanocages has been established. Well-defined polyelectrolyte nanocages have been obtained as one-to-one precise polymerized copies of the surfactant monolayers absorbed on crystallized nanodroplets. This method for the synthesis of nanocages via “crystal-forming” emulsions can lead to more accurate templating control than conventional emulsion-based approaches, without involving tedious procedure typically required in the approach of nanoparticle excavation. Having unique structures with monolayer-thick amphiphilic shell, these polyelectrolyte nanocages potentially may have broad applications in drug delivery, phase transfer catalysis, nanoreactor, and lay-by-layer preparation of complex nanomaterials.