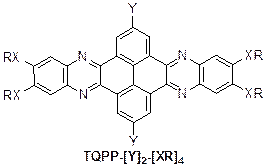www.acsprf.org
Reports: B1047343-B10: Design and Synthesis of Novel Pyrene Discotics and Their Investigation in Organic Photovoltaic Cells
Bilal R. Kaafarani , American University of Beirut
During the third year of this project, we continued exploiting the discoid molecules based on quinoxalino[2',3':9,10]phenanthro[4,5-abc]phenazine TQPP-[Y]2-[XR]4, Figure 1, for potential electronic applications.
Figure 1. Chemical Structure of TQPP-[Y]2-[XR]4.
The synthesis of three novel TQPP derivatives was achieved (Y = t-Bu, XR = SPhytanyl; Y = 4-t-Bu-phenyl; XR = SC12H25; SPhytanyl). The UV and fluorescence spectra of these compounds were similar. This indicates the absence of conjugation between the aryl ring and the TQPP core.
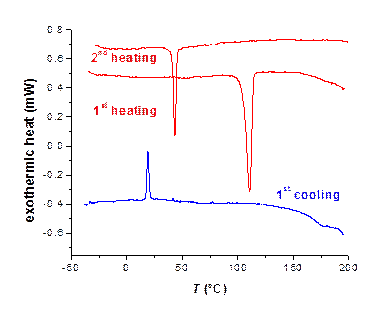
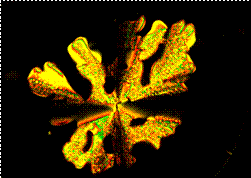
TQPP-[4-t-Bu-phenyl]2-[SPhytanyl]4 displays columnar liquid crystal phases. The initial phase obtained from solution is a soft crystal phase of probably tilted columnar structure that irreversibly melts into a hexagonal columnar (Colh) mesophase at 105 °C, Figure 2. The Colh clears into the isotropic liquid phase at 239 °C, which was observed only by POM not by DSC. On cooling the Colh is stable down to 21 °C when it reversibly converts into a tilted (likely rectangular) columnar liquid crystal phase, Figure 3.
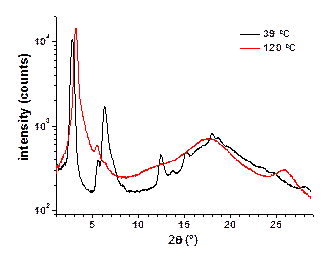
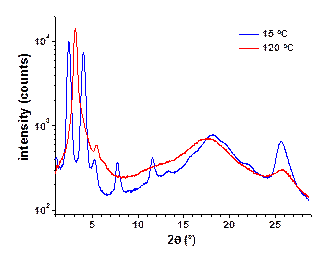
Both, diffraction data (Figure 3) and POM investigations (Figure 4) of TQPP-[4-t-Bu-phenyl]2-[SPhytanyl]4 unambiguously verify the assignment of the high temperature mesophase as a Colh phase. The small angle diffraction peaks can be indexed as (10), (20), and (11) reflections that together are characteristic for a hexagonal phase. A calculated intercolumnar spacing of about 3.24 nm based on the (10) reflection is a reasonable value if compared to an average diameter of 3.6 nm for the molecule with extended side-chains. The halo of the aliphatic side-chains at about 0.45 nm confirms a molten state of the side-chains and the peak at about 0.35 nm confirms the presence of p-p stacking. Dendritic growth textures observed by POM are characteristic for a hexagonal columnar mesophase and the presence of pseudoisotropic areas also confirms the uniaxial character of this phase, Figure 4.
Figure 2. DSC heating (10 °C/min) and cooling (5 °C/min) curves of TQPP-[4-t-Bu-phenyl]2-[SPhytanyl]4 under N2. No reversible transitions were observed at temperatures between 200 °C and 260 °C (not shown).
Figure 3. Diffraction patterns of TQPP-[4-t-Bu-phenyl]2-[SPhytanyl]4 at 39 °C before heating (soft crystal phase), upon heating to 120 °C (Colh), and after cooling to 15 °C (probably Colr).
Figure 4. POM micrograph of the Colh phase of TQPP-[4-t-Bu-phenyl]2-[SPhytanyl]4 between glass slides at 230 °C on cooling from the isotropic liquid at 1 °C/min (crossed polarizers, 200x magnification). Dark areas around the dentritic texture are homeotropically aligned.
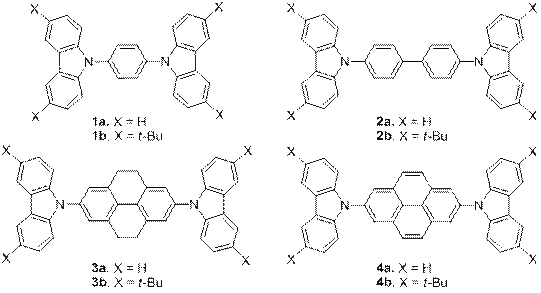
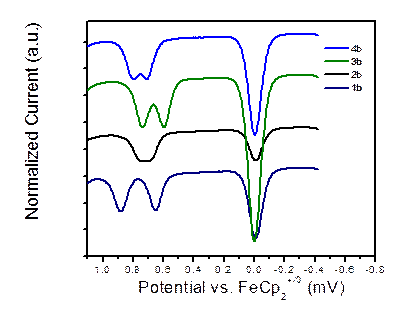
Furthermore, we synthesized a series of carbazole derivatives 1-4 to be exploited in organic-light emitting diodes, Figure 5. The absorption and emission data of 1-4 are presented in Table 1. Only di-tert-butyl carbazole derivatives (i.e. 1b, 2b, 3b, and 4b) showed ideal reversible redox behavior composed of two sequential oxidation processes (while scanning in the positive direction) followed by symmetric reduction peaks. The reversible oxidation processes make these compounds as potential hole carriers. DPV of 1b, 2b, 3b, and 4b are shown in Figure 6. On the other hand, the non-symmetric redox behavior of the carbazole derivatives (i.e. 1a, 2a, 3a, and 4a) is accompanied by broadening of the oxidation peaks, possibly due to the instability of the oxidized carbazole moieties and the degradation of the compound in solution. The oxidation process for these compounds was not reversible since the electrochemically active sites of carbazole (C3 and C6) were not blocked, thus resulting in electrochemical polymerization of carbazole.
Figure 5. Structures of carbazole derivatives.
Table 1. Absorption and emission maxima for 1-4 in DMF.
Compound
| Bridge
| Substituent
| λabsmax (nm)
| λemmax (nm)
|
1a
| benzene
| carbazole
| 301
| 363
|
1b
| benzene
| 3,6-di-tert-butylcarbazole
| 312
| 371
|
2a
| biphenyl
| carbazole
| 322
| 387
|
2b
| biphenyl
| 3,6-di-tert-butylcarbazole
| 328
| 404
|
3a
| 4,5,9,10-tetrahydropyrene
| carbazole
| 339
| 381
|
3b
| 4,5,9,10-tetrahydropyrene
| 3,6-di-tert-butylcarbazole
| 350
| 389
|
4a
| pyrene
| carbazole
| 306
| 429
|
4b
| pyrene
| 3,6-di-tert-butylcarbazole
| 321
| 450
|
Figure 6. Differential Pulse voltammograms (50 mVs-1) for bis(3,6-di-tert-butylcarbazol-9-yl) derivatives: 1,4-bis(di-tert-butylcarbazol-9-yl)benzene (1b), 4,4'-bis(di-tert-butylcarbazol-9-yl)biphenyl (2b), 2,7-bis(3,6-di-tert-butylcarbazol-9-yl)tetrahydropyrene (3b), and 2,7-bis(3,6-di-tert-butylcarbazol-9-yl)pyrene (4b). Ferrocene was used as an internal standard in CH2Cl2/0.1 M nBu4NPF6.
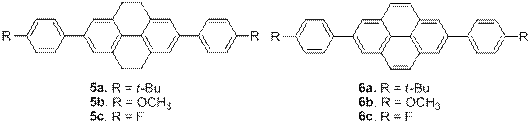
Furthermore, we synthesized two series of 2,7-disubstituted 4,5,9,10-tetrahydropyrenes (5a-c) and pyrenes (6a-c) carrying steric factor, electron withdrawing and electron donating substitutent, Figure 7. More than twice higher melting points and bathochromic shifts in absorption and fluorescence were observed as a result of the substitutions. Both series exhibited solvent-dependent quantum yields and lifetimes. X-ray crystallographic analysis of all six compounds revealed edge-to-face packing and the absence of weak intermolecular interactions in the solid state.
Figure 7. Pyrene and tetrahydropyrene derivatives.