www.acsprf.org
Reports: UR550095-UR5: Adsorption of CO2 by MgO Aerogels for Enhanced Steam Reforming
Mingheng Li, PhD , California State Polytechnic University
Adsorption Enhanced Reforming (AER) is a novel steam reforming technique that enables the production of fuel-cell grade hydrogen in a single unit. The key feature of this process is the removal of CO2 from the reactive system using an adsorbent, so that the reforming and water gas shift reactions shift to the right, resulting in an enhancement of the yield and purity of H2. The central objective of the project is to investigate MgO aerogle as a promising CO2 adsorbent for AER applications. The report covers the period during 9/1/1010-8/31/2011 where the focuses are adsorption capacity measurement using thermogravimetric analyzer (TGA) and breakthrough curve measurement using a small packed-bed reactor.
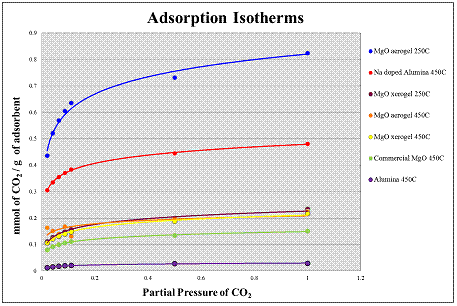
In the TGA experiment, the weight gain of material samples exposed to different CO2 partial pressures was measured. In all the experiments the samples were heat treated at two different temperatures They were either heat treated at 250 oC or 450 oC for 4 hours and then at 250 oC for another 4 hours, all under N2. Subsequently, at a constant temperature of 250 oC, CO2 was introduced first at 2% relative to N2, then at 4%, 6%, 8%, 9%, 50% and then finally 100%. Lastly the CO2 was shut off for another 8 hours in order to allow the material to desorb the CO2 adsorbed during the experiment. The material studied where: (1) MgO aerogel heat treated at 250 oC and 450 oC, (2) MgO xerogel heat treated at 250 oC and 450 oC, (3) commercial MgO heat treated at 450 oC, (4) Alumina heat treated at 450 oC, and (5) Na doped Alumina heat treated at 450 oC.
Figure 1: Adsorption Isotherms of material studied. |
The TGA data showed that the MgO aerogel heat treated at 250 oC had the highest adsorption capacity compared to all the material studied. The reason for this very high adsorption capacity could be attributed to the very high surface area of the aerogels that possibly increased the number of oxygen sites available for the CO2 to adsorb on. As compared to commercial MgO, the adsorption capacity of the MgO aerogel was 7 times higher in terms of CO2 adsorption capacity. This was the result of both high surface area and amorphous structure combined. In order to study the effect of structure alone the MgO xerogel was compared with the commercial MgO due to their similarity in surface area. It was shown that the CO2 adsorption capacity of the MgO xerogel was about 2 times higher than the adsorption capacity of the commercial MgO.
A packed bed reactor has been constructed with instrumentation and control to study adsorption/desorption of CO2 under more realistic conditions. Testing has been done to study the CO2 breakthrough curve of commercial Na promoted Alumina:
|
Figure 2: Breakthrough curve of CO2 on Na promoted alumina in a packed bed reactor.
|
Ongoing experimental studies are being pursued in order to study how the MgO aerogel will perform in this reactor. Such experiments will show the performance of MgO aerogel during continuous adsorption/desorption cycles under realistic conditions. The studies will also suggest the reactor size needed in order for this process to take place.