www.acsprf.org
Reports: DNI450341-DNI4: Experimental Study of Oxidation Mechanisms of Large Hydrocarbons by the Photolytic Formation of Key Radical Intermediates
Jesse Kroll, PhD , Massachusetts Institute of Technology
Project Goals and Activities. This project is aimed at gaining an improved understanding of oxidation mechanisms of complex hydrocarbons. Such reactions play key roles in several chemical processes important to energy, industry, and the environment, including combustion of fossil fuels, weathering of organic materials such as oil and asphalt, environmental degradation of pollutants, and formation of secondary atmospheric species such as tropospheric ozone and secondary organic aerosol (SOA). The goals are to gain an improved understanding of the chemistry of two key radical species – alkylperoxy radicals (RO2) and alkoxy radicals (RO) – by forming these intermediates not by reactions of organics with oxidants but rather by photolysis of radical precursors. Such an approach avoids much of the drawbacks associated with initiating reactions with gas-phase oxidants, the typical approach for studying hydrocarbon oxidation processes. These drawbacks include interferences from multiple reaction channels, multiple oxidation generations, and competing chemistry, all of which are minimized when the radicals are generated directly. Two approaches are used for generating radicals: the 254 nm photolysis of alkyl iodides (RI) in the presence of oxygen to form RO2 radicals, and the ~350 nm photolysis of alkyl nitrites (RONO) to form RO radicals.
In Year 1 of this project we carried out a number of experiments involving both types of radicals (RO and RO2). These experiments were carried out in two different reactors, chosen based on the light source available. Studies of RO radicals were carried out in the PI's environmental chamber, which is surrounded by blacklights that emit in the UV-A (300-400 nm, overlapping well with the absorption spectrum of RONO), whereas studies of RO2 radicals were carried out in a flow reactor surrounded by UV-C (254 nm) lights for the efficient photolysis of RI. As discussed below, we have successfully demonstrated that the photolysis of both classes of radical precursor in the gas-phase leads to the formation of condensed-phase (particulate) products, and that the amounts and chemical composition of these products can afford insight into the underlying mechanism of the oxidative chemistry.
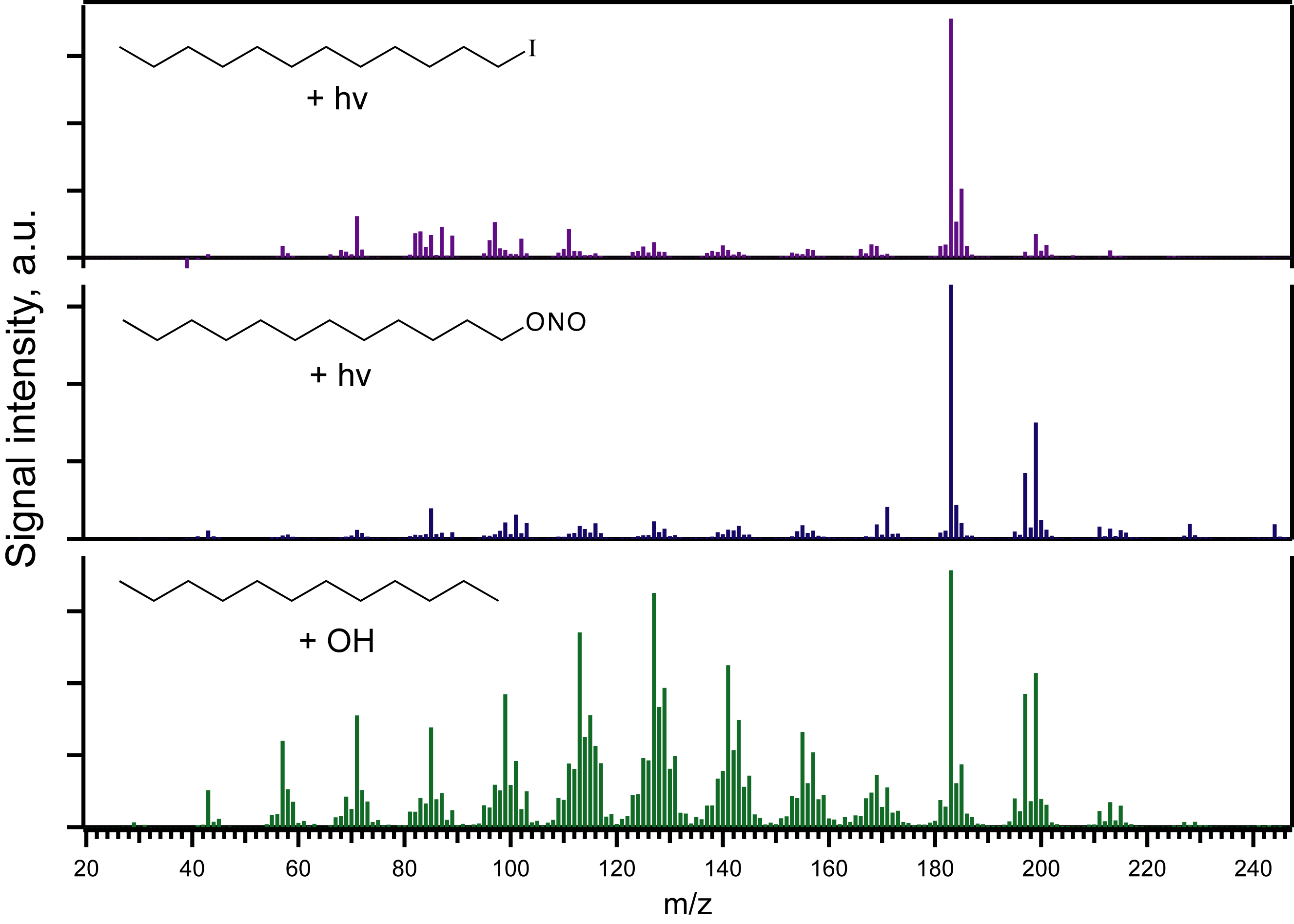
Results: first-generation oxidation of n-alkanes. The focus of Year 1 of this project has been studying the initial molecular products formed in the oxidation of one of the most important (and simplest) classes of hydrocarbons, n-alkanes. We have found that large (C7 or greater) alkyl iodides and alkyl nitrites both form aerosol upon photolysis, indicating the formation of low-volatility products. This formation of fine particles is useful from an analytical perspective, in that it enables the use of aerosol mass spectrometry (AMS), a technique for the quantitative introduction of analyte into a mass spectrometer. (PRF-DNI funding went towards the purchase of an AMS for this project.) Shown in Figure 1 is a comparison of the aerosol mass spectra of particles formed from various reaction systems associated with n-dodecane oxidation: UV-C photolysis of dodecyl iodide (top panel), UV-A photolysis of dodecyl nitrite (middle panel), and OH + dodecane (bottom panel). The three spectra exhibit a number of key similarities (such as the large peak at m/z 183, likely corresponding to a furan species), but large differences are obvious also. Most importantly, mass spectra from the photolytically-generated aerosol (top two panels) are substantially "cleaner" (more chemically tractable) than that of the oxidant-initiated aerosol (bottom panel), confirming the utility of this approach in understanding detailed chemical mechanisms. More subtle differences (such as the high-mass peaks in the top two panels, and the O:C and H:C ratios of the aerosol) also provide insight into the differences in reaction pathways taken.
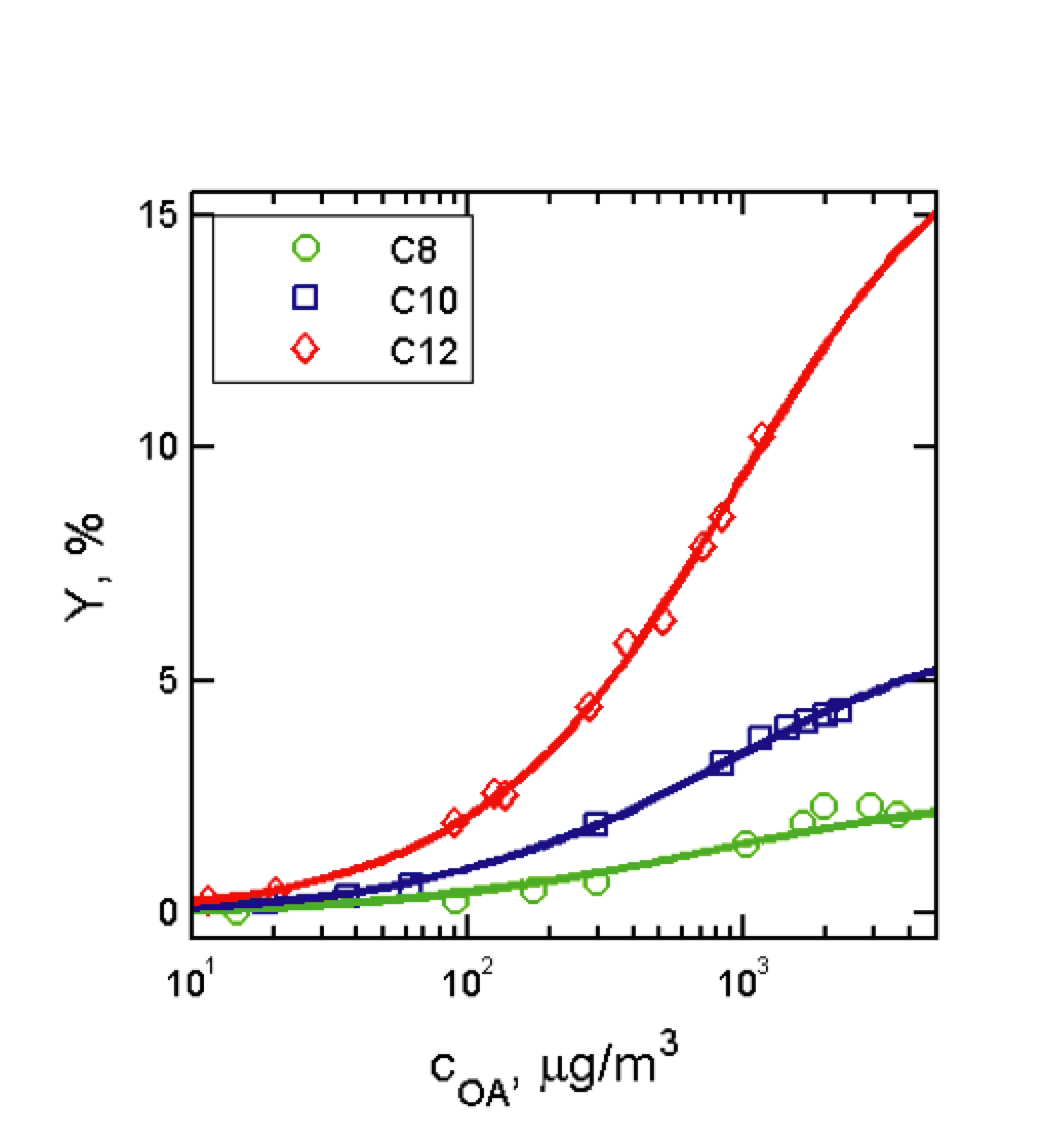
The amounts of aerosol formed also yields information about the product distributions. "Yield curves" (aerosol mass yield vs particle mass loading) for the 254 nm photolysis of 1-iodoalkanes (iodooctane, iododecane, and iodododecane) are shown in Figure 2. The yields of aerosol generated from the photolysis of alkyl iodides were found to depend not only on carbon number (as expected), but also on aerosol loading, indicating the semivolatile nature of the species formed. However, the aerosol measured was found to have lower yields than in previous multigenerational studies of alkane oxidation, suggesting that further oxidative steps produce additional semivolatile material in those experiments (as well as in many real-world systems).
The photolytic formation of individual radical species enables the study of individual reaction pathways, which generally cannot be done in oxidant + hydrocarbon studies. We have synthesized several isomeric alkyl nitrites (heptyl-1-nitrite, heptyl-2-nitrite, heptyl-3-nitrite, and heptyl-4-nitrite), for the study of the role of radical position (the carbon number on which the alkoxy center is located) on product formation. Results indicate that the primary radicals (from heptyl-1-nitrite) product significantly more aerosol that the secondary ones; comparison of mass spectra suggest this is mostly a result of structure effects on vapor pressure rather than differences in chemistry.
Planned Work in Year 2. Having demonstrated that this general approach – the photolytic formation of large alkyl radicals and the study of the resulting aerosol products – enables detailed study of the mechanisms of the (relatively well-understood) n-alkane oxidation system, we plan to extend it in Year 2 to more complex chemical systems. We have already carried out several experiments examining the low-volatility (aerosol) products of the photolysis of iodobenzene, in order to investigate the products of the oxidation of aromatic species. In these studies substantial aerosol production was observed; the close analysis of those data is a major goal of the following year. We then hope to extend this approach to still other hydrocarbon skeletons, including those with branches, rings, and double bonds, to gain a detailed understanding of the role of these moieties in governing oxidation mechanisms. Further, we will extend studies beyond purely gas-phase oxidation processes, by photolyzing condensed- (particle-) phase alkyl nitrites. This will allow for the detail investigation of the chemistry of condensed-phase alkoxy radical species, which at present are highly uncertain.