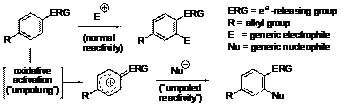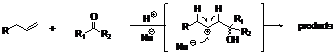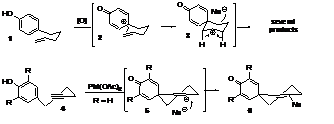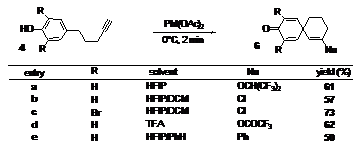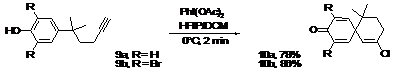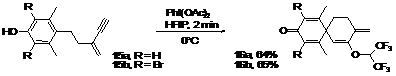www.acsprf.org
Reports: B148122-B1: Cycloaddition Reactions for the Formation of Indolines, Indoles and Benzofurans, and Subsequent Functionalization Reactions
Sylvain Canesi , Universite du Quebec a Montreal
This research report focuses on the accomplishments achieved during the second year of this grant:
The central theme of our research is what may be termed "aromatic ring umpolung." Whereas an electron-rich aromatic nucleus normally reacts as a nucleophile, suitable oxidative activation can convert it to a reactive electrophilic intermediate, which may be intercepted with external nucleophiles, Scheme 1. Our research focuses on the capture of such intermediates with carbon nucleophiles, leading to the formation of new C-C bonds. Among the various reagents that could be used in the oxidative step,hypervalent iodine complexes were found to be the agents of choice. Figure 1.
This method requires no metal catalysts, it does not need activated substrates, and it is environmentally benign. This inversion of polarity generates a phenoxonium ion 2 allowing to transpose cationic transformation in aliphatic chemistry to the aromatic chemistry via an oxidative process. Such an approach has been investigated to perform an oxidative Prins process on phenol derivatives. The well-known Prins transformation was first reported by Kriewitz. The reaction involves addition of an oxonium species to an alkene or an alkyne followed by capture of a nucleophile or elimination of hydrogen. Since there are several competing reaction pathways, it is important to control the reactivity of the electrophilic species generated in order to obtain only the desired product, Figure 2.
One control method was proposed by Overman et al. in the famous Prins-pinacol tandem process. This method considerably reduces the formation of byproducts from the electrophilic intermediate generated and often forms an efficient key step in the synthesis of complex natural products. We were interested to extend this famous transformation to phenols, the feasibility of this transformation was first investigated on compound 1. If the desired compound was well observed, as expected, the desired compound was accompanied by a mixture of products representing the different plausible pathways beginning with such an intermediate 3. To limit the formation of side products, the reaction was investigated using a free alkyne moiety 4 as the nucleophile. It was rationalized that the geometry of the strained half-chair species 5 would strongly favor nucleophile capture, leading to a spiro[5.5]undecanyl core 6, Figure 3.
The strain generated by the transient sp-hybridized carbon in species 6 rendered the intermediate highly electrophilic and enabled it to react with weakly reactive nucleophiles, including some normally used as inert solvents such as HFIP, dichloromethane (DCM) (as a chloride donor), trifluoroacetic acid (TFA), and benzene. This last example can be considered as a tandem oxidative Prins/Friedel-Crafts process, demonstrating the potential utility of this strategy for further domino applications, Table 1.
To determine the scope and limitations of this novel transformation, various substituents were introduced on the lateral chain to generate more elaborate bicyclic systems. A summary of experiments performed using 1- or 2-substituted phenols is presented in Table 2.
Substitution at position 2 (R2) seems to have no influence on the yield of this reaction. However, the presence of bromine atoms in the ortho positions or more importantly a substituent in position 1 (R1) clearly increases the yield. This result could be rationalized by considering that a common limitation of oxidative processes is competitive hydrogen elimination during formation of the phenoxonium ion, leading to an easily polymerizable quinone methide. The presence of only one hydrogen atom would limit this side reaction. In addition, the weak electron donating effect of the substituent in position 1 could slightly increase the stability of the phenoxonium ion, favoring nucleophilic addition. These results suggest the potential for constructing highly hindered cores containing contiguous quaternary carbon centers. Indeed, the elaboration of such challenging systems is often restricted by the steric hindrance of the first quaternary carbon center. The introduction of two substituents in position 1 would help stabilize the phenoxonium ion generated during the umpolung activation and would create a contiguous spiro quaternary center. The difficulty normally associated with the synthesis of such frameworks would turn into an advantage. To verify this hypothesis a second substituent was introduced at position 1, we were pleased to observe the formation of the desired compounds in good yields considering the architecture produced. Scheme 1.
We have also investigated the effect of a methyl group in position 3 and the formation of a spiro[4.5]decanyl core 14 via a Wagner-Meerwein transposition process has been observed. The electron density provided by the methyl group in position 3 probably favored a ring contraction process on the species 12. The corresponding carbocation 13 was trapped by various nucleophiles present in the medium to afford compound 14, Scheme 2.
This transposition may be easily avoided by introducing a methylene group in position 3; indeed, the sp2 hybridization in this position now retards the ring contraction process in favor of nucleophilic capture. In addition, methyl groups were introduced at the meta positions, Scheme 3.
In summary, a new oxidative Prins process has been developed that enables rapid access to functionalized and highly hindered spirocyclic compounds such as those present in numerous natural products. This method is an efficient means of producing two contiguous quaternary carbon centers.