www.acsprf.org
Reports: DNI549261-DNI5: Surface Organometallic Chemistry for Improved Performance and Understanding of Hydrodenitrogenation Catalysis
Justin M. Notestein, PhD , Northwestern University
Hydrotreating is the essential process of removing N and S from crude oil and other fuels. As the U.S. moves to crudes with higher heteroatom content, there is a significant need for fundamental understanding of hydrotreating catalyst design and mechanisms. Hydrodenitrogenation (HDN) has been studied less and is particularly energy inefficient due to the high temperatures required and high H2 consumption. Therefore, research was proposed to synthesize and study model HDN catalysts using Ta and Nb surface organometallic chemistry. Ta and Nb are chosen due to intriguing C-C, N-N, C-N cleavage activity by surface and soluble organometallic complexes. On surfaces, these isolated cations are also expected to only begrudgingly saturate aromatic rings, which is a challenge to the H2 efficiency of existing processes. In addition to specific insights into HDN, new syntheses are developed for supported catalysts in general, particularly group 4 and 5 supported catalysts.
To date, research has focused on supported Ta catalysts. Several new catalysts have been synthesized, characterized, and tested in alkene epoxidation (published) and HDN (report ready for publication). A student has been placed on this project; the work described here is partially supported by ACS PRF, internal Northwestern University fellowships, and a self-funded postdoctoral fellow who has now left the group.
SYNTHESIS AND CATALYTIC TESTING.
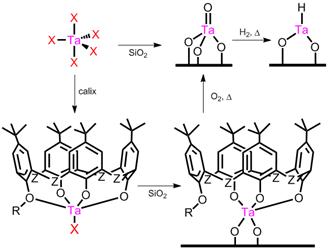
Following the proposal, TaX5 complexes have been grafted to silica and alumina as controls under inert conditions via alcoholysis or simple ligand exchange. Calixarene-Ta complexes have also been grafted by adding stoichiometric calixarene in toluene to TaCl5 or Cp*TaCl4, refluxed, and then previously dried support is added to the same vessel. (Figure 1) Intermediate complexes are not isolated because reactions are known to be high yielding. Ligand tert-butylcalix[4]arene is used primarily, but others have been explored.
FIGURE 1. Synthesis of supported Ta catalysts.
These catalysts have been tested for alkene epoxidation with H2O2. This requires highly isolated surface cations, which is also desired for our HDN catalysts, making this a relevant probe. The details of these reports, and the uniqueness of the calixarene ligand, are described in two recent publications citing ACS PRF support.
For this proposal, these catalysts have been tested in HDN of quinoline in dodecane, modeling recalcitrant N-containing molecules in crude oils. Reactions were carried out at approximately 40 bar H2 from 275 to 350°C.
Calixarene-Ta supported on SiO2 or Al2O3 were either directly calcined or first impregnated with Pd(OAc)2, then calcined, and compared to Ta-containing catalysts from more typical precursors (i.e. TaCl5, TaBz5). Prior to use in HDN, the calcined catalysts were reduced in H2 in the reactor at 350°C to 500°C. Key results are that: 1) highly dispersed Ta is able to carry out hydrogenation and hydrodenitrogenation, with products that depend on the support, 2) under typical reaction conditions (up to 350°C, 40 bar H2), the catalyst is not reduced, but pretreatments above the onset of reduction (~400°C) increase arene hydrogenation at the expense of the desired hydrodenitrogenation activity, 3) as proposed, isolated Ta removes N atoms without complete saturation, to form in this case, propylcyclohexene and propylbenzene, wheras supported Pd exclusively produces propylcyclohexane, albeit at higher yields, 4) conversion to denitrogenated products can be increased dramatically, while still retaining some selectivity to partially unsaturated products by depositing Pd onto the Ta-modified supports, and 5) co-locating the Pd and Ta on the same surface is critical, as is use of the highly dispersing calixarene precursors.
As hypothesized, these catalysts show reactivity different from that typically observed in HDN and may offer a new route to lower H2 consumption. The mechanism and surface intermediates in this transformation are now the focus of study, as well as expanded empirical testing. It is believed that these catalysts are capable of carrying out direct C-N cleavage from sp2-hybridized carbons and/or are effective deamination catalysts to yield alkenes. This work is forming the basis of a major research area in the group of the PI.
CHARACTERIZATION.
The calcined d0 Ta(V) materials have been characterized by diffuse reflectance UV-visible spectroscopy (DRUV) and XAS at the Advanced Photon Source, which confirm the presence of highly isolated Ta(V). Temperature programmed reduction shows the onset of reduction in H2 at ~400°C; consistent with this, neither DRIFTS nor XAS of samples previously reduced to 350°C (reaction conditions) show evidence of a reduced site or a Ta-H species. Likewise, the used catalyst (protected from air), shows no evidence for any catalyst restructuring to a carbide, nitride, or any significant change to the first coordination sphere of Ta.
IMPACT.
This funding has been used to collect preliminary data for proposals submitted to the National Science Foundation and is the basis for a new collaboration (joint PhD student) with a professor in the Chemistry department. This grant has provided supplies support for a postdoc with an external fellowship, and enabled partial support of a student in the first year to explore a new area of research related to this proposal.