www.acsprf.org
Reports: DNI550443-DNI5: Adaptable and Smart Amphiphilic Systems in Enhanced Oil Recovery
Mustafa Akbulut, PhD , Texas A&M University
NARRATIVE REPORT
Introduction
Typical enhanced oil recovery (EOR) techniques aim to achieve one or more of the following: (i) decrease oil viscosity, facilitating the movement of oil within porous oil-bearing rock (for example, by thermal flooding); (ii) increase the viscosity of water to more effectively sweep oil out from the deposit (for example, with viscosity-modifying polymers); and (iii) reduce capillary forces or interfacial tension between oil and the oil-bearing stratum (for example, with surfactants). This proposal aimed (i) to develop novel EOR techniques involving 'adaptable', and 'smart' amphiphilic systems to achieve these properties and (ii) to understand the physical chemistry, thermodynamics, and material and surface science critical for the design and operation of such systems.
Results
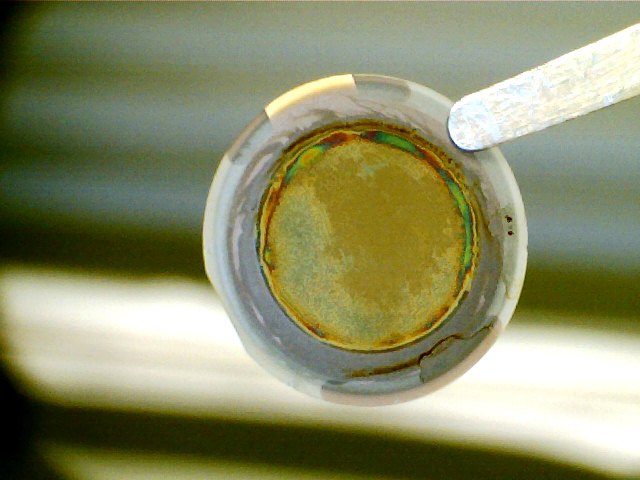
We relied on the quartz crystal microbalance with dissipation (QCM) technique to in situ characterize the structure, mass, viscoelastic properties of the oil-SiO2 interface that is exposed a surfactant solution. First, we found that one of the most important steps for obtaining reproducible results in this system is the formation of robust thin film of oil (asphalt). For instance, depending on the method of preparation, oil can dewet the SiO2 sensor (i.e. forms oil droplets on the sensor), or completely delaminate from the sensor. The evaporation of asphalt/tetrahydrofuran mixture under vacuum condition on the sensor led to fairly smooth and robust asphalt film (Fig. 1).
Figure 1. (a) Blank SiO2 QCM sensor. (b) Asphalt coated SiO2 QCM sensor.
The initial total mass of the asphalt film on the sensor was measured by calculating the frequency change of QCM-D relative to the blank sensor. Then, while the coated sensor was exposed to 4-nonylphenyl- polyethylene glycol (non-ionic surfactant) solution of various concentrations; frequencies, f and dissipations, D were monitored at several harmonics (15, 25, 35, 45, 55, and 65 MHz) simultaneously. All measurements in the flow chamber were performed at a temperature of 25°C, to within 0.1°C to avoid drifts in f and D. The analysis of the QCM frequency shifts as a function of time allowed us to calculate the total mass of asphalt as a function of surfactant solution exposure time (Fig. 2). Figure 2 shows that the asphalt mass decreased continuously with increasing time of exposure. In addition, the rate of removal was larger at higher concentrations while the removal process was inefficient below critical micelle concentration of surfactant.
Figure 2. The total mass of asphalt on the SiO2 sensor as a function of exposure time to the surfactant solution.
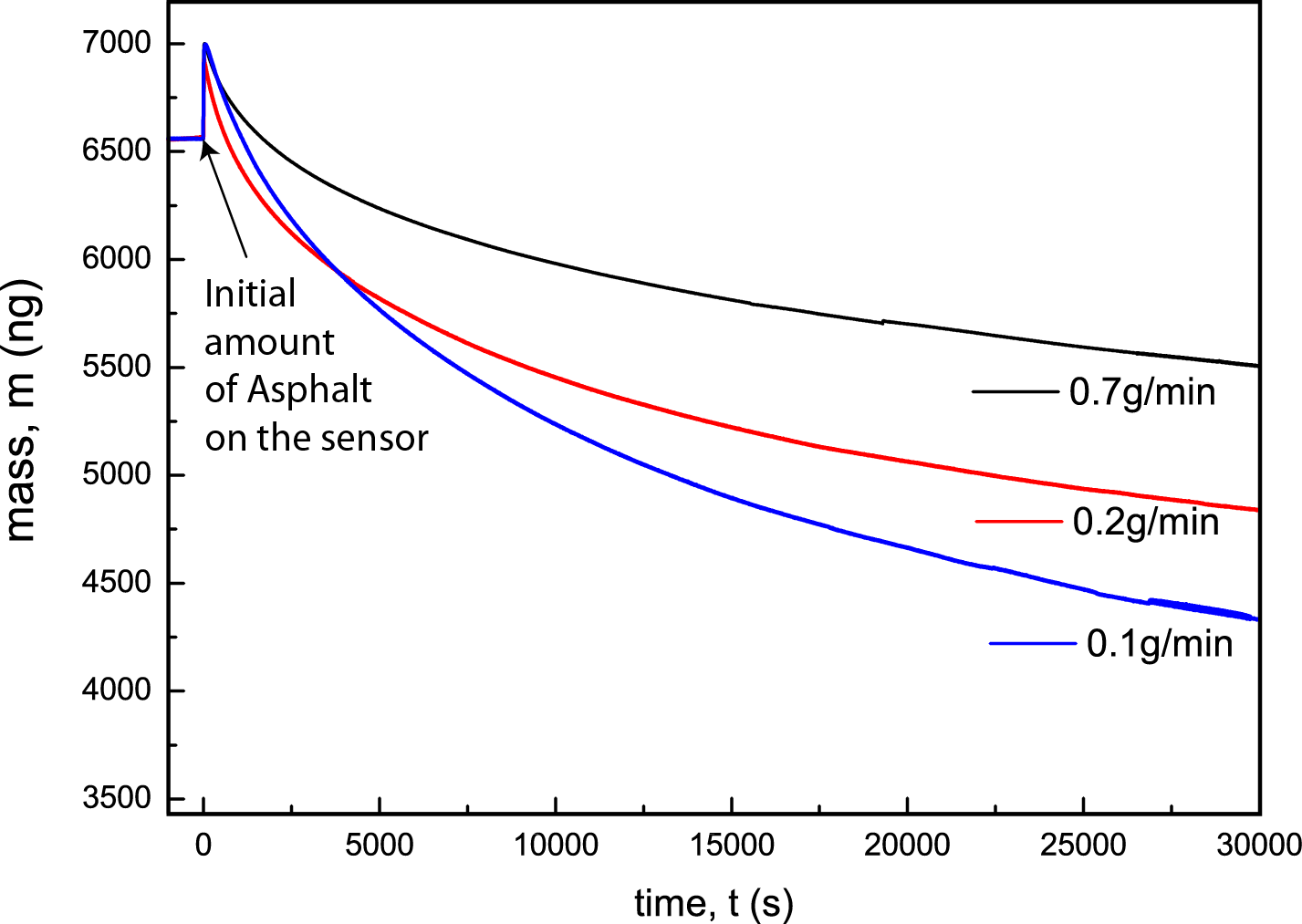
We have also investigated the effect of flow velocity of surfactant solution on the rate of asphalt removal (Fig. 3). It was found that the removal rates were smaller at larger flow rates. This is presumably because large flow rates the surfactant solution does not have enough time to interact with the asphalt layer on the SiO2 sensor.
Figure 3. The total mass of asphalt on the SiO2 sensor as a function of flow rate of surfactant solution.
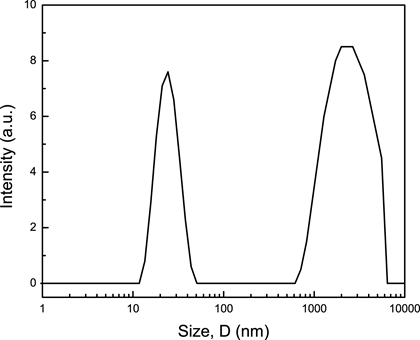
In addition, the effluent of QCM experiments were analyzed by dynamics light scattering (DLS). The particle size distribution data shows that there are two main peaks: one ranging from 600 nm to 7 mm and the other ranging from 10 nm to 50 nm (Fig. 4). The larger particles indicate emulsions and the smaller particles indicate empty surfactant micelles. The presence of emulsions confirms that 4-nonylphenyl- polyethylene glycol can be used to remove asphalt which is adsorbed on a silica surface.
Figure 4. Intensity weighted particle size distributions for the QCM effluent which was formed by flowing a 4-nonylphenyl- polyethylene glycol over asphalt layer adsorbed on a silica surface.
Regarding the synthesis aspect of the project, we are currently synthesizing an amphiphilic block copolymers containing poly(2-vinylpyridine) described in Scheme 1.
Scheme 1
Future work
In future studies, we seek obtain a better understanding of how dynamics of assembly/disassembly and adsorption/desorption processes of newly developed adaptable and smart amphiphiles depend on specific amphiphilic properties, such as absolute and relative sizes of hydrophobic and hydrophilic groups, and molecular structure (branching, linearity, and aromaticity) and modes of activation such as temperature, and pH.