www.acsprf.org
Reports: DNI749218-DNI7: Assembly of Organometallics in Polymeric Templates for Metal Coated Mesoporous Materials
Bryan D. Vogt , University of Akron
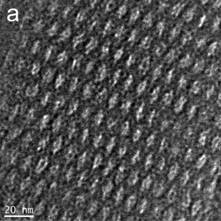
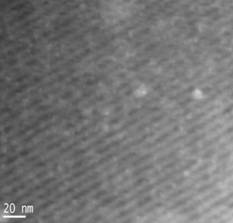
This work focuses on understanding the assembly of surfactants, carbonizable precursors and organometallic compounds to fabricate nanoporous composite materials that have potential use in catalysis and energy storage (batteries/supercapacitors). In the past year, we have focused on a non-reactive system based upon cooperative assembly of a non-ionic surfactant, a phenolic resin and an organometallic to create these hybrid structures. Figure 1 illustrates the well ordered composite structures that can be obtained and the high quality dispersion of nanoparticles, in this case CoO. The oxidation state for these nanoparticles was determined by EDS and XPS.
Figure 1. HR TEM image (left) of porous carbon-cobalt oxide film formed cooperative assembly route Z-contrast STEM (right) is utilized to demonstrate the dispersion of cobalt oxide nanoparticles (small white dots) in the ordered carbon matrix. |
One interesting observation is that the addition of small concentrations of the cobalt oxide can dramatically increase the electrical conductivity of the porous carbons, which is now attributed to graphitization of the carbon induced by the transition metal. Further increases in concentration of the metal oxide nanoparticles leads to a decrease in conductivity as would be expected for the addition of insulating nanoparticles. This increased conductivity should be beneficial for electrochemical applications. Figure 2 illustrates the electrochemical capacitance for mesoporous carbons that contain CoO nanoparticles as a function of cycling. Without any added nanoparticles, the capacitance is quite low (~24 F/g), but is stable through 500 cycles. Addition of CoO nanoparticles initially dramatically increases the initial capacitance of the material to greater than 100 F/g. However at higher loadings (>10 wt % CoO), there is a substantial decrease in the capacity after only several cycles. The lower loading sample exhibits a very stable capacitance through 500 cycles. We attribute the differences in the performance to the size of the CoO nanoparticle inclusions. For sub-10 nm particles, the capacitance is relatively stable; while for larger nanoparticles, there is a substantial drop in the capacitance during the first several cycles. This has been confirmed by using VO2 nanoparticles.
Figure 2. Electrochemical capacitance of CoO nanoparticle containing mesoporous carbons. CCo-10, CCo-20 and CCo-33-800 contain 4.7, 17.5, and 25.3 wt % CoO, respectively. FDU-16-800 is the pure carbon (no CoO) references. |
We have also extended this work to powder samples. Interestingly, the increase in the amount of carbon around the nanoparticle during carbonization leads to full reduction to metallic nanoparticles. For electrochemical applications, this reduction to metal is undesired, but Co nanoparticles should be highly magnetic and could be useful for separations. We have recently demonstrated the ability of these mesoporous carbons to act as adsorbents for n-butanol recovery in biofuel applications as illustrated in Figure 3. The equilibrium uptake is similar to the standard commercial porous polymer, but the kinetics are significantly improved. Additionally, the mesoporous carbons are highly stable in aqueous solutions and have high thermal stability, which are important characteristics for in-situ product recovery.
Figure 3. Adsorption kinetics for dilute n-butanol from aqueous solution for (●) mesoporous carbon and (■) traditional porous polymers. |
These concepts can be applied to other systems as well. We have recently shown that size exclusion principles can be utilized in the separation of methylene green from aqueous solution. If the mesopores are too small, the dye is not accessible to the large internal area and almost no appreciable adsorption occurs. As the pore size increases by several nm, the dye can be fully removed from solution. This provides a potential route for selective separations in aqueous waste streams by variation in the mesopore size. However, recovery of the mesoporous carbons can be difficult. With the addition of cobalt nanoparticles, the materials can be readily recovered magnetically as illustrated in Figure 4.
Figure 4. Adsorption of methylene green from aqueous solutions using mesoporous carbons with different average sizes (top); the pore size increases from left to right by ~ 2 nm and demonstrates the potential for fine control in bulky molecule separations from aqueous streams. These powders containing cobalt nanoparticles can be used to remove the dye and then be separated from suspension magneticially. |