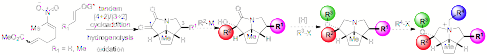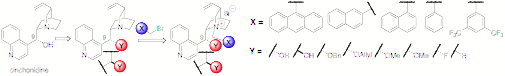www.acsprf.org
Reports: ND149668-ND1: A Systematic Evaluation of Chiral Phase Transfer Catalysts
Scott E. Denmark, DScTech , University of Illinois (Urbana-Champaign)
Progress Report
I.Statement of Objectives. The primaryobjective of this application was to elucidate the factors that govern thestructure-reactivity-selectivity relationships in asymmetric phase transfercatalysis (ATPC) with chiral quaternary ammonium ions. This program willinvolve an iterative process that involves the synthesis, evaluation,physicochemical analysis, and design of the chiral quaternary ammoniumions. These activities will berepeated for the optimization of the catalyst structure.
Thefirst phase of the program involved the design and development of two differentchiral scaffolds for the quaternary ammonium salts for application inasymmetric phase transfer catalysis. The [4+2] cycloaddition and tandem[4+2]/[3+2] cycloaddition of nitroalkenes are highly convergent processes thatallow the assembly of rigid, polycyclic amine scaffolds that can be embellishedwith a variety of different functional substituents in a convergent region ofspace. Two different chiralscaffolds will be investigated that present substituent in a differentarrangement and introduce them in a different sequence. Parallel synthesismethods will be employed to generate large libraries of ammonium ions fortesting.
Thechiral ammonium salts will be evaluated for their potential to catalyze anumber of synthetically important transformations and a QuantitativeStructure-Selectivity Profile will be developed to explain the roles of thedifferent substituents.
II.Cyclopentapyrrolizidinium Library Synthesis and Data Collection. In May 2011, back-to-back Feature Articles werepublished that detailed over 5 years of work (ca. 100 journal pages and >600 SI pages) on the synthesis, evaluation, and QSAR/QSSR modeling ofcyclopentapyrrolizidinium catalysts. Over 160 ammonium salts were synthesizedthat contained varying numbers of substituents at positions R1-R4as well as different configuration at C(1). The substituents allowedvarious effects to be evaluated such as steric and electronic contributions, p-surface, lipophilicity, and polar surfacearea on the catalyst structure. The rate data for the O'Donnell benzylation(50% aq. KOH/toluene) covers four orders of magnitude of activity (t1/2from 5 min to over 15 h) and was deemedsuitable for model development. In addition we determined the stir-ratedependence over a range of stir rates (1038 – 2500 rpm). Importantly,there is a sufficient range of stirring speeds, along which the activities ofall of the catalysts surveyed are dependent on mixing rate (i.e. operate in thetransport-limiting regime).
Theenantioselectivity for the chiral catalysts was largely dependent on thesubstituents R2 and R4. The enantioselectivity isgreatest for catalysts that bear (1) strongly electron withdrawing groups onthe nitrogen, (2) a p-surface at the R2substituent, and (3) an alkyl group at R1.
Scheme 1
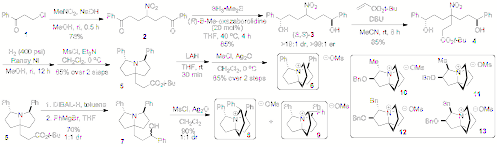
III.Azoniapropellane (AZP) Based Catalysts. Separateroutes to two families of enantiomerically enriched 1-azoniapropellanes havebeen developed that differ in the location of substituents proximal to theammonium nitrogen. The most efficient and diversifiable is illustrated inScheme 2. Double Michael addition with nitromethane with 1 afforded nitro diketone 2 in good yield. Corey-Itsuno reduction affordedexcellent selectivity of the chiral diastereomer (S,S)-3 in high enantiomeric purity. Michael addition with tert-butylacrylate followed by reduction of the nitro group and mesylation of thealcohols led to spontaneous closure to pyrrolizidine 5. LiAlH4 reduction of the ester followedby mesylation gave the disubstituted AZP's 6. Alternatively, DIBAL-H reduction followed by in situreaction of the aldehyde with phenylmagnesium bromide afforded a mixture ofcarbinols, 7. Mesylation of themixture afforded a separable mixture of C3 and C1AZP's, 8 and 9, resp. Four other azoniapropellanes (10-13)were synthesized in enantiomerically enriched form by the second route.
All sixAZP's (9 was unstable to opening) weretested for their potential as APTC's in the O'Donnell alkylation. All of thesalts showed good catalytic activity (t1/2 ranged from11 min to 420 min) but poor enantioselectivity (up to 60:40 er). Molecularmodeling and ESP surfaces suggest that the 8 may be too congested above the top face. However, as outlined in thenext section, the topography of these ammonium salts lends itself much moresensibly to APTC reactions wherein the stereogenic center is created on theelectrophile
Scheme 2
IV. Cinchona Alkaloid-Based Catalysts. In a recently completed study, the results ofsystematically varying two positions of cinchonidinium salts was reported. A39-member catalyst library containing various N-quinuclidiniumsubstituents (X) and C(9) substituents(Y) was prepared and the rateand selectivity for catalyzing the O'Donnell alkylation was evaluated (Scheme3). Significant conclusions could be drawn from these data. First, the rate ofthe alkylation is strongly dependent on both the X and Y substituents,but not on the configuration. Of the X substituents, 1-naphthyl is the fastest and 3,5-(CF3)2C6H3is the slowest. Of the Y substituents,O-alkyls are the fastest and OH, F and H are the slowest. Theenantioselectivity is primarily dependent on the N-quinuclidiniumsubstituent X and independent of theC(9) substituent and its configuration.
Scheme3
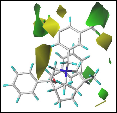
V.Enantioselectivity Model Development. To develop a CoMFA model with sufficientpredictive capacity (Q2LOO > 0.6) it was necessary toemploy different conformational representations of the catalysts. As a result,an optimal model could be developed with good correlation (Chart 1a). From rigorouscross-validation protocols the model proved to be fairly robust accounting forits statistical relevance.
Chart 1
The qualitative application of an MIF model may berealized by the visual inspection of the relative magnitudes of thecoefficients of the regression model and may be represented as colored contours.Chart 6b shows the steric contours: green (↑ steric → ↑e.r.); yellow (↓ steric → ↑ e.r.) and Chart 6c showselectrostatic contours: blue (↑ (+)-charge → ↑ e.r.); red(↓ (+)-charge → ↑ e.r.). The contours are confluent withqualitative observations including the requirement for decreased steric bulk onthe left face, increased steric bulk on the right face, electron-withdrawinggroup at R4=3,5-(CF3)C6H3, and p-surface at R2 (increasedpositive potential). The contour maps have revealed valuable insight withrespect to the region of the catalyst to which the enolate should associate forgreater enantioselectivities.