www.acsprf.org
Reports: DNI149965-DNI1: Catalytic Scaffolding Ligands: An Efficient Directing Group Strategy
Kian L. Tan, PhD , Boston College
With the support of the PRF over the past year, we have expanded our scaffolding concept to both metal and non-metal catalyzed reactions. The research effort has been split between finishing several publications in the area of hydroformylation as well as starting up a new project in the area of organocatalysis.
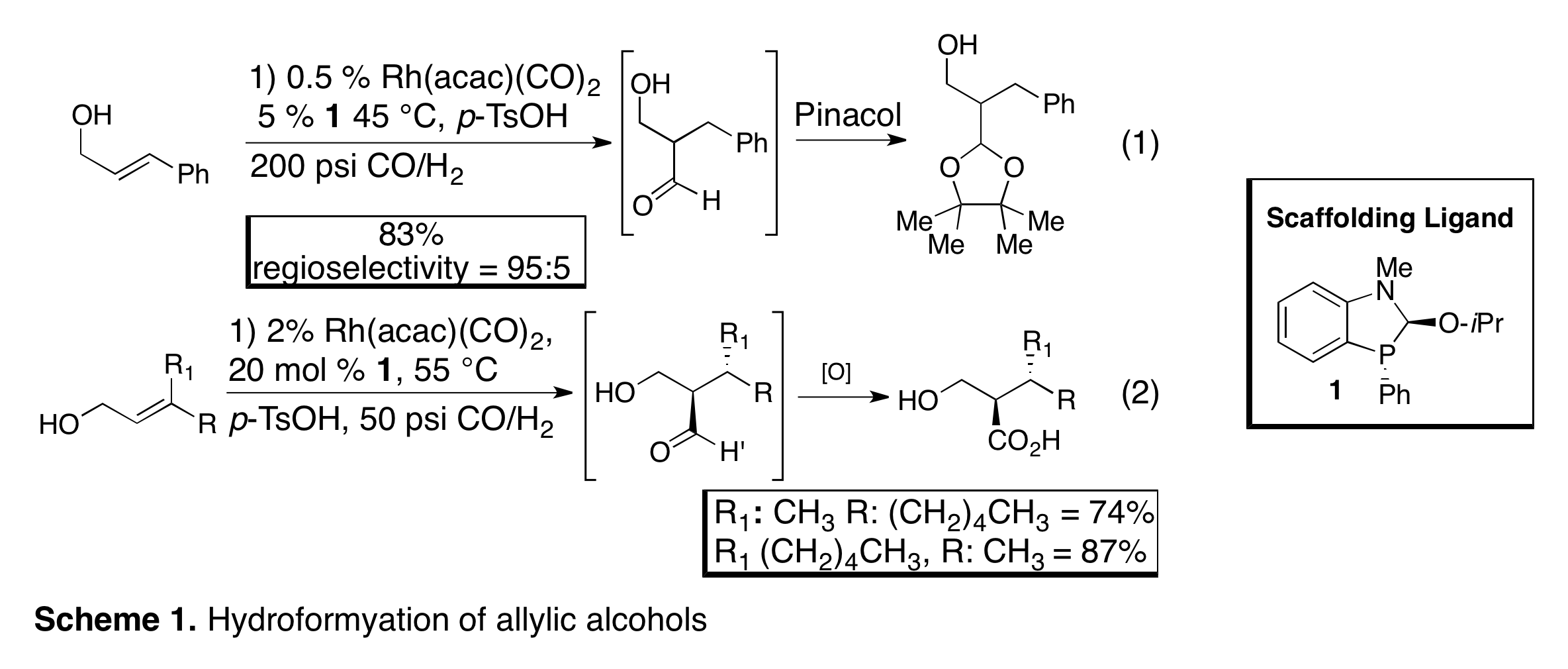
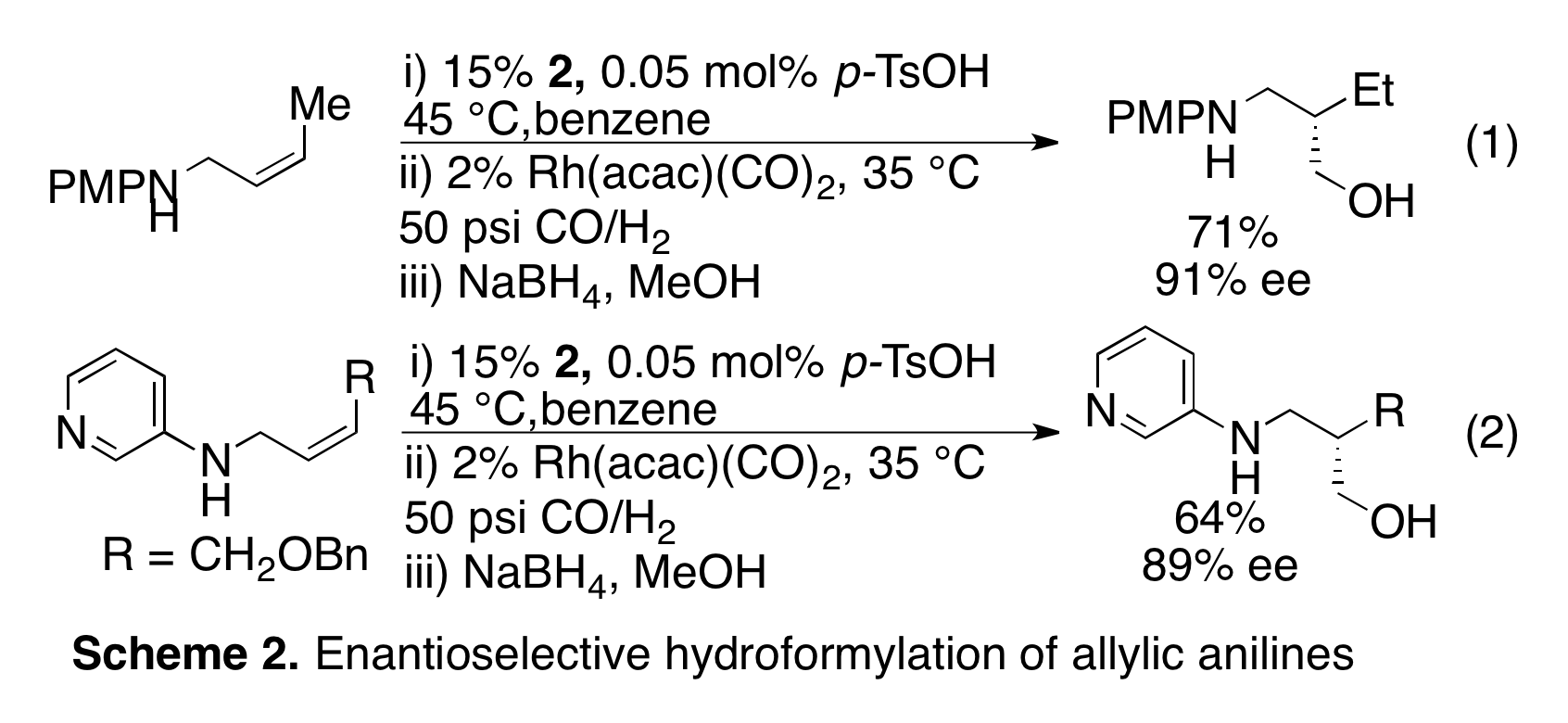
As noted in last year's progress report we had discovered that hydroformylation of 1,1-disubsituted olefins with 1 leads to the regioselective hydroformylation to form quaternary carbon centers. This research effort was brought to completion and published in the J. Am. Chem. Soc (2010, 132, 11841). Notably this paper was highlighted in Chemical & Engineering News, (2010, 33, p. 41), because of the challenge of forming quaternary carbon centers through hydroformylation. We further demonstrated that terminal, 1,2-disubstituted, and trisubstituted olefins can be hydroformylated with 1 to form the b-hydroxy-aldehyde products (Scheme 1, Org. Lett. 2011, 13, 2686). We also published in J. Am. Chem. Soc. (2010, 132, 14757) the first enantioselective hydroformylation using a catalytic directing group (Scheme 2, eq 1). We applied ligand 2 in the asymmetric hydroformylation of allylic amines towards the synthesis of b-amino-aldehydes. Hydroformylation of (Z)-5 with 2 yields the desired b-amino alcohol in good yield and up to 91% ee (Scheme 2, eq 1). The optimized conditions are very mild (35 °C and 50 psi CO/H2), demonstrating the power of directing groups to accelerate reactions. More recently we have continued to investigate substrate scope for enantioselective hydroformylation. In a follow up to the hydroformylation of p-methoxy aniline derivatives, we have discovered that a range of anilines can undergo highly enantioselective hydroformylation, including electron-deficient heterocycles such as pyridine (Scheme 2, eq 2). We further found that the binding affinity to the ligand is affected by electronic perturbations to the nitrogen with electron rich nitrogens' binding more strongly to the ligand than electron deficient. This affinity also correlates with general stability of the ligands such that ligands bound to electron-rich anilines decompose at a slower rate. This work was recently accepted for publication in the J. Org. Chem.
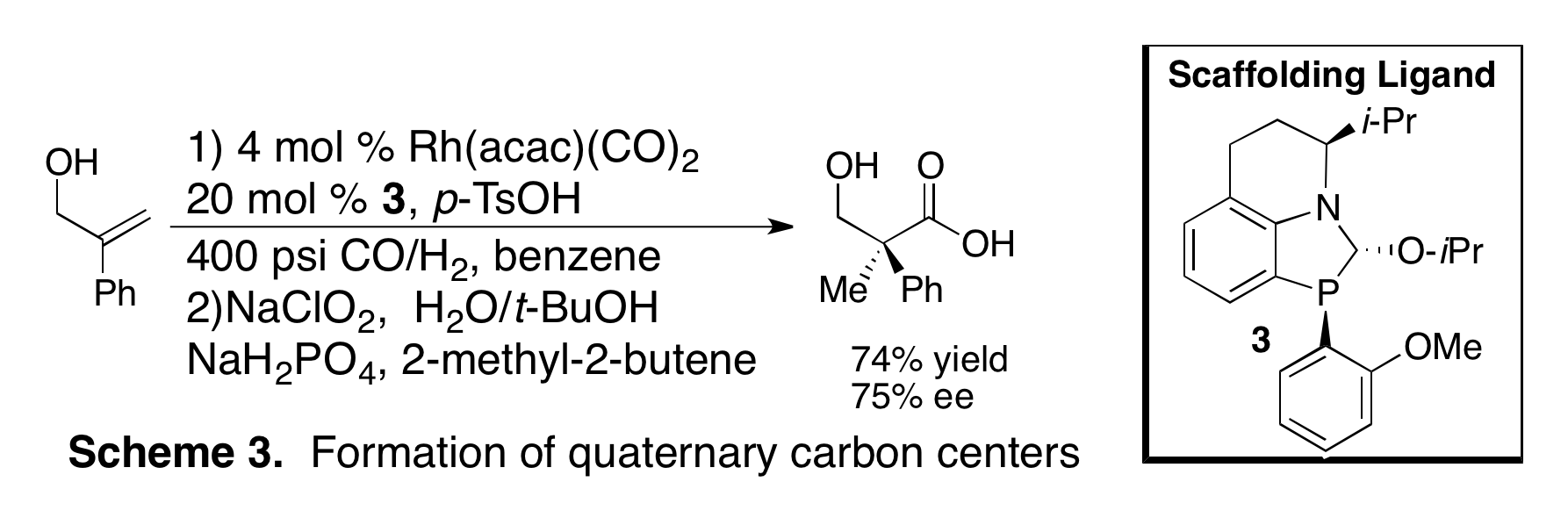
Initial attempts to perform the enantioselective hydroformylation of allylic alcohols to form quaternary carbon centers resulted in low enantioselectivity. We synthesized ligand 3, a derivative of 2 with an o-OMe-Ph group, that improves the enantioselectivity to 75% ee (Scheme 3). We are currently pursuing this promising lead by making new ligand derivatives as well as investigating new ligand designs.
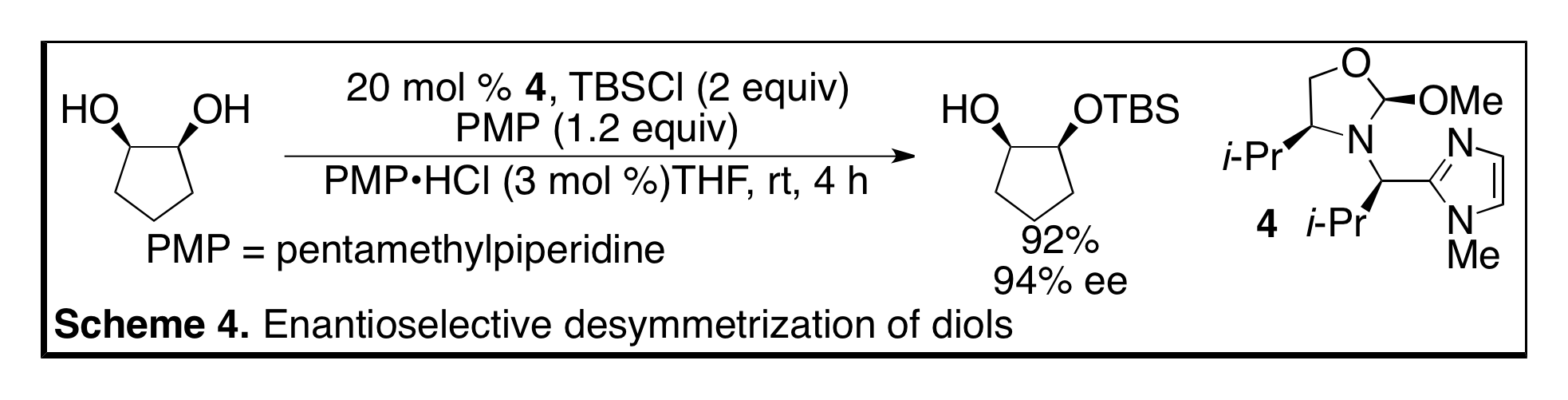
In a significant step forward for the concept of scaffolding catalysis, we redesigned our catalyst to incorporate an imidazole group instead of a phosphine. Whereas the phosphine serves as a ligand for a metal we envisioned that the imidazole could act as an organic catalyst. To test this idea we investigated the desymmetrization of 1,2-diols via silylation. After optimization of the catalyst structure we found that catalyst 4 afforded both high enantioselectivity and yield in the desymmetrization of cis-1,2-cyclopentanediol (Scheme 4). The substrate scope was extended to heterocycles, medium-sized rings as well as acyclic substrates (Angew. Chem. Int. Ed., 2011, early view). We are continuing to investigate the utility of these catalysts; in particular we are applying the catalysts to the functionalization of complex molecules such as sugars and a natural products.
In conclusion this past year we have addressed a significant problem in hydroformylation, the formation of quaternary carbons. Furthermore, we have developed an enantioselective catalyst system for the asymmetric hydroformylation of allylic amines. We have extended the concept of scaffolding catalysis to organic catalysts towards the highly enantioselective desymmetrization of 1,2-diols.