www.acsprf.org
Reports: UNI650291-UNI6: Computational Design of CO2-Philic Hydrocarbon Polymers to Promote More Efficient Oil Recovery
Collin Wick, PhD , Louisiana Tech University
PRF Progress Report
The work carried out in the first year of the PRF grant has focused on understanding water-alkane interfaces. This was a slight departure of the investigations of the alkane-carbon dioxide mixtures, including the influence of additives. The overall goal of the proposed research was to build a fundamental understanding of enhanced oil recovery methods, and the fact that a much more common method for this, steam injection, is not well understood, encouraged me to investigate this system first. Simple questions such as how the effect of alkane chain length influence the structure, how the alkane-water interface differs from the air-water interface, and the influence of oil oxidation on their interfacial properties with water, are not well understood. Because of this, I deemed organic-water interfaces were worth investigating first. This work was presented at an invited talk at a major conference, and has been written up in a publication that has been submitted. This work was carried out by one undergraduate student and a graduate student. The undergraduate student will present some of the results of this work for a CAPSTONE project, which is required to get an ACS certified degree from Louisiana Tech University.
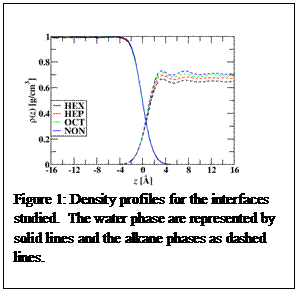
We carried out molecular dynamics simulations of the water-hexane (HEX), water-heptane (HEP), water-octane (OCT), and water-nonane (NON) interfaces. To model these interfaces, the simulation box was elongated in the z-direction so that two water-alkane interfaces were formed bisecting the z-axis. A new molecular model was developed for the alkanes that included polarizable interactions, which allow induced dipoles to form in response to an electric field. Polarizable interactions are important for describing interfaces, as changes in density often results in changes in the local electric field, and if the molecular model is polarizable, its structure can change more significantly at interfaces. Figure 1 gives the density profiles for the systems investigated with zero representing the Gibbs dividing surface (GDS) of water. The densities away from the interfacial region were close to their bulk experimental values. Furthermore, the surface tensions of the varying interfaces agreed well with experiment, which were some of the experimental results used to validate the new molecular models. Of further interest is that as the alkyl chain length got longer, the alkane-water interfacial widths decreased. This was in contrast to x-ray reflectivity experiments [1]. This disagreement with experiment is being investigated further to determine if it is because of systems size effects of the simulations, and is important to understand. For instance, capillary waves are known to become more important at larger lateral boxlengths (or the boxlengths parallel to the interface). Does capillary wave theory, which is required to link molecular simulation results with experiment, describe this adequately [2-4]? Furthermore, the alkane density profiles in figure 1 show clear oscillation to regions far away from the interface, which is consistent for all of the systems studied. This could mean that a larger alkyl region in the longitudinal direction (perpendicular to the interface) could also influence the interfacial width. These all are factors that need to be understood before a complete description of the alkane-water interface can be achieved.
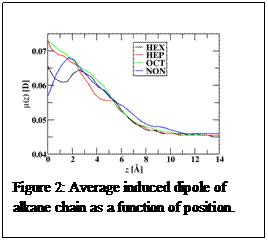
Due to the fact that the molecular model included polarizable interactions, the molecular dipole of water and the alkane molecules depend on their local environment. One consequence of this can be observed in figure 2, which shows the alkane induced dipole as a function of position with zero representing the GDS of water. It can be observed that the alkane induced dipole increases as the alkane chains approach the interfacial region. Water, on the other hand, showed a decrease in induced dipole as they approached the interfacial region (results not shown). This result makes sense in that the water phase is more polar than the alkane phase, which should induce dipoles from the alkane phase in the interfacial region. In contrast, since the alkane phase is less polar than the water phase, water molecules near the alkane have a lower induced dipole. This results in the water phase expanding at the interface. These insights are novel and due to the fact that the molecular model includes polarizability.
References
[1] D. M. Mitrinović, et al., "Noncapillary-wave structure at the water-alkane interface," Physical Review Letters vol. 85, pp. 582-585, 2000.
[2] I. Benjamin, "Molecular structure and dynamics at liquid-liquid interfaces," Annual Review of Physical Chemistry vol. 48, pp. 407-451, 1997.
[3] F. P. Buff, et al., "Interfacial density profile for fluids in the critical region," Physical Review Letters vol. 15, p. 621, 1965.
[4] J. S. Rowlinson and B. Widom, Molecular Theory of Capillarity. New York: Oxford University Press, 1989.