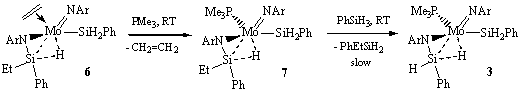Reports: AC3
45990-AC3 New Silanimine and NSi-H-M Agostic Complexes for Coupling with Petroleum Derived Products
The specific results of the current grant period are:
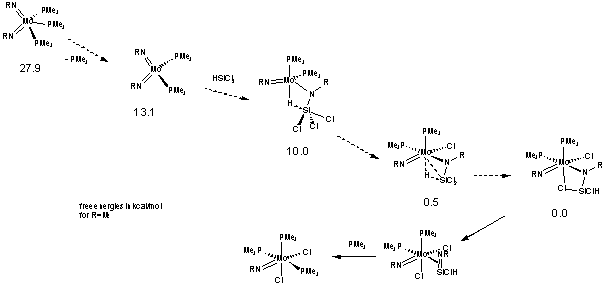
1) The reaction of (RN)2Mo(PMe3)3 with HSiCl3 has been earlier reported to furnish (RN)2MoCl2 (PMe3)3 and the silanimine dimer (RNSiClH)2. We carried out detailed investigation of this reaction for R = Ar (Ar=2,6-Pri2C6H3), 2,6-Me2C6H3, But, and found that the first observable product (at -30 °C) is the Cl-bridged species (ArN)(k2-ArNSiHCl-Cl )Mo(PMe3)2Cl (1). Analogous compound is formed and can be isolated when the W complex (ArN)2W(SiCl2Me)(H)(PMe3) reacts with PMe3. Back to Mo, warming the reaction mixture to 15 - 0 °C leads to a slow rearrangement of this initial product into another bis(phosphine) compound whose spectroscopic parameters are consistent with the silanimine (ArN)(η2-ArN=SiHCl)Mo(PMe3)3Cl2 (2). Addition of a further equivalent of PMe3 to the latter gives (ArN)2MoCl2(PMe3)3 and the dimer (RNSiClH)2, thus proving the inference of a silanimine ligand. Detailed DFT calculations of the reaction between the model complex (MeN)2Mo(PMe3)3 and silanes HSiMenCl3-n (n=1-3) underpin our mechanistic conclusion (Scheme 1). A report on this chemistry has been accepted to Inorganic Chemistry and is selected as a cover paper for an October issue 2009.
Scheme 1.
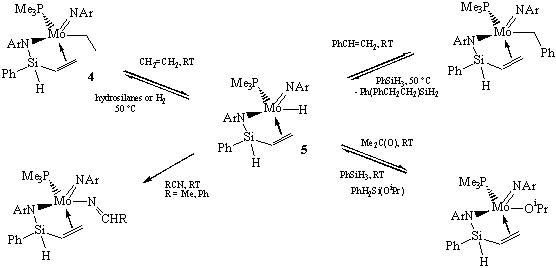
2) We continued investigation of the reactivity of the agostic complex (ArN=)(η3-ArN-SiHPh-H...)Mo(SiH2Ph)(PMe3) (3), and its derivatives. In particular, we carried out detailed mechanistic study of the reaction of complex (ArN=)(ArN-SiHPh-CH=CH2)Mo(CH2CH3)(PMe3) (4), formed upon the reaction of 3 with ethylene, with silanes in order to determine the mechanism of olefin hydrosilylation. The formation of the first product, the hydride (ArN=)(ArN-SiHPh-CH=CH2)Mo(H)(PMe3) (5), is overall a dissociative reaction (DS≠<0) but detailed kinetic measurements revealed a complex interplay of two competing mechanisms (Scheme 2).
Scheme 2.
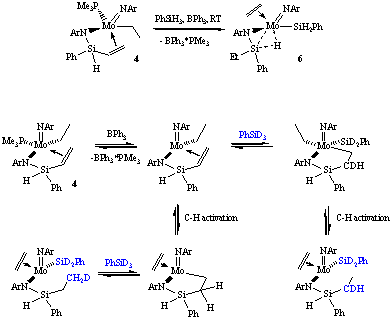
When the exchange reaction was attempted in the presence of BPh3, the reaction takes a different path and gives a Si-H agostic ethylene derivative (ArN=)(η3-ArN-SiEtPh-H...)Mo(SiH2Ph)(CH2=CH2) (6), in which the ethyl group on the agostic silicon results from the vinyl group hydrogenation and the olefin ligand comes from a b-H shift from the ethyl. A possible mechanism of this transformation supported by labelling experiments is shown in Scheme 3. Subsequent reaction of 6 with PMe3 affords the agostic complex (ArN=)(η3-ArN-SiEtPh-H...)Mo(SiH2Ph)(PMe3) (7),which then slowly reacts with the silane to give the starting compound 3, thus closing the cycle. The mechanism of the latter transformation is still under investigation.
Scheme 3.
We also investigated the reactivity of the hydride species (ArN=)(ArN-SiHPh-CH=CH2)Mo(H)(PMe3) (5) that is very prone to insertion reactions, as summarized in Scheme 4.
Scheme 4.
3) Our initial plan to prepare silanimine complexes supported by tripodal ligands isolobal with Cp, did not yield the target compounds. However, while pursuing this goal, we came across some interesting compounds exhibiting catalytic activity. Thus, we carried out detailed mechanistic investigations on the reaction of complex Cp(ArN=)Mo(H)(PMe3) (8) with H3SiPh and on the mechanism of carbonyl hydrosilylation catalyzed by 8. Kinetic measurements, labelling experiments, and DFT calculations of the first reaction established a s-bond metathesis mechanism for the formation of Cp(ArN=)Mo(SiH2Ph)(PMe3) (9). This result is significant because s-bond metathesis reactions are not typical for d2 systems, such as complex 8. The catalytic hydrosilylation mediated by 8 proceeds as a two-step reaction, starting with the unexpected for a 18e complex associatively activated insertion of carbonyl into the Mo-H bond (for PhCH=O: DH≠ = 59.4 kJ/mol, DS≠ = - 93 J/(K*mol)) to give Cp(ArN=)Mo(OR)(PMe3) (10) followed by an associatively activated reaction of the Mo-O bond with silane (DH≠ = 58.3 ± 4.8 kJ/mol, DS≠ = -113 ± 15.9 J/(K*mol)) to regenerate 8. Such a reaction should be classified as a heterolytic Si-H cleavage. To the best of our knowledge, this is the first detailed mechanistic investigation of this type of mechanism.
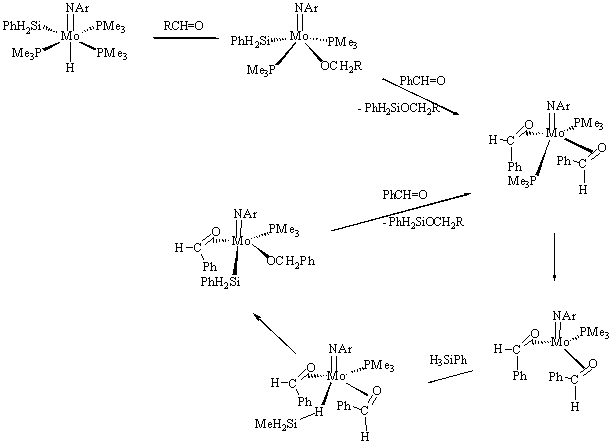
4) The new complex (ArN=)(Me3P)3Mo(H)(SiH2Ph) (11) was accidentally prepared in the course of our research on silanimine compounds described in section 2. Complex 11 was found to be an efficient catalyst for a variety of hydrosilylation reactions (carbonyls, alkenes, alcohols, nitriles). Mechanistic investigations revealed that its reactions with ketones give the alkoxy derivatives (ArN=)(Me3P)2Mo(H)(OR) (12, Scheme 5). With PhCH=O, however, a low temperature NMR study detected (ArN=)(Me3P)2Mo(h2-PhCHO)2 as the first observable product. This compound easily releases phosphine to give the bis(aldehyde) species (ArN=)(Me3P)Mo(h2-PhCHO)2 (13) which was isolated and studied by NMR and X-ray diffraction. DFT studies show that 13 adds silane to give an unusual h1-silane s-complex (MeN=)(Me3P)Mo(h2-MeCHO)2(h1-H3SiMe) (14), which then transfers the hydride to aldehyde to give the intermediate (MeN=)(Me3P)Mo(h2-MeCHO)(OCH2Me)(SiH2Ph) (15), in a step preceding the formation of the hydrosilylation product.
Scheme 5.
The current PRF grant has made a profound effect on boosting our research program in molecular catalysis, particularly in that part which involves hydrosilylation reactions mediated by silanimine and Si-H agostic compounds. New Si-C and Si-N bond formation and Si-N bond cleavage reactions have been discovered, along with new and unexpected reaction pathways. During the current grant period, the students involved in the project learned new NMR techniques (such as 1D EXSY) to study kinetics of chemical reactions and became very well trained in sophisticated mechanistic research.