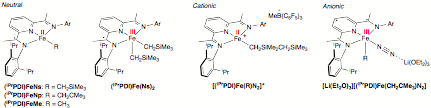Reports: AC3
46425-AC3 Understanding Iron Catalyzed Olefin Polymerization
Aryl-substituted bis(imino)pyridine iron and cobalt dichloride complexes, (ArPDI)MCl2 (M = Fe, Co) when activated with methylaluminoxane (MAO), exhibit high activities for ethylene and a-olefin oligomerization and polymerization. Since the initial, independent reports in 1998 by Brookhart and Gibson, many studies have focused on alteration of the modular bis(imino)pyridine ligand framework to establish structure-reactivity relationships. For the iron compounds, pre-catalysts bearing two large 2,6-substituents on the aryl ring are known to produce linear polyethylene whereas those with only a single ortho aryl substituent are selective for a-olefin production with near ideal Schultz-Flory distributions.
Despite a fairly mature understanding of how catalyst structure influences the type of oligomer or polymer produced, the mechanism of olefin polymerization and the nature of the active species formed upon treatment of (ArPDI)FeCl2 with MAO remain controversial. NMR studies indicate formation of both Fe(II) and Fe(I) compounds albeit with two active species, while EPR and Mssbauer spectroscopic studies support Fe(III). During the PRF funding period, we have addressed these issues by preparation of well-defined compounds and evaluation of their performance in catalytic olefin polymerization. From studies such as these, we aim to develop a mechanistic understanding of the polymerization reaction with the goal of synthesizing new catalysts with improved performance.
During the past funding period efforts continued to elucidate the electronic structure (e.g. determine the true iron oxidation state) of bis(imino)pyridine iron alkyl complexes. To systematically address this issue, a family of neutral, cationic and anionic compounds (Figure 1) was prepared a studied by a combination of X-ray diffraction, Mssbauer spectroscopy and magnetochemistry. A summary of Mssbauer parameters is presented in Table 1. The experimental work has been augmented by open shell, broken symmetry DFT calculations where the computational results were calibrated by comparison to experimental metrical data from X-ray diffraction and Mssbauer parameters.
Figure 1. Neutral, Cationic and Anionic Bis(imino)pyridine Iron Alkyl Compounds Relevant to Olefin Polymerization.
Table 1. Summary of Zero-field Mssbauer Parameters Recorded at 80 K for Neutral, Cationic and Anionic Bis(imino)pyridine Iron Alkyl Compounds.
|
Compound
|
d (mm/sec)
|
DEQ (mm/sec)
|
|
(iPrPDI)Fe(CH2SiMe3)2 |
0.27
|
2.65
|
|
(EtPDI)Fe(CH2SiMe3)2
|
0.27
|
2.65
|
|
|
|
|
|
(iPrPDI)Fe(CH2CMe3)
|
0.57
|
1.16
|
|
(iPrPDI)Fe(CH2SiMe3)
|
|
|
|
|
|
|
|
[(iPrPDI)Fe(CH2SiMe3)(Et2O)]+ |
0.88
|
2.22
|
|
|
|
|
|
[(iPrPDI)Fe(CH2CMe3)N2]-
|
|
|
|
[(iPrPDI)Fe(CH2SiMe3)]-
|
0.06
|
0.86
|
|
[(iPrPDI)Fe(C6H4-4-Me)N2]-
|
0.02
|
0.93
|
The findings from these studies were illuminating in light of the previous work in the field. Both classes of neutral compounds – the mono and dialkyl derivatives – have a ligand centered radical and hence a monoanionic bis(imino)pyridine. Thus, both Fe(II) and Fe(III) alkyl complexes are possible. Upon protonation or alkyl abstraction from the iron dialkyl, a redox process occurs at both the ligand and the metal, the ligand becomes oxidized to its neutral form and the ferric ion is reduced to ferrous. Analysis of the data for the anionic complex, [(iPrPDI)Fe(CH2CMe3)N2]- establishes that one electron reduction of the neutral compound to the anion results in a two electron reduction of the ligand and an oxidation of the iron! These detailed spectroscopic and computational studies firmly establish the ability of the bis(imino)pyridine iron complexes to smoothly adjust their electronic structure in response to various reaction conditions and serve to highlight why there has been such confusion in the literature as to the oxidation state of the propagating species.
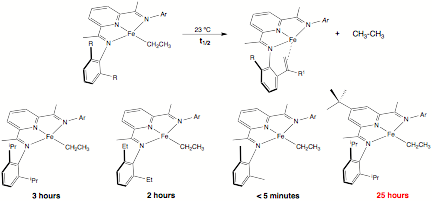
We have also continued to investigate fundamental transformations relevant to olefin polymerization and discovered some surprising effects of ligand substitution on rates of b-hydrogen elimination, an important chain transfer pathway. As reported last year, the bis(imino)pyridine iron ethyl complex, (iPrPDI)FeCH2CH3, persists in benzene-d6 solution at 23 ¼C with a half life of 3 hours. Transfer dehydrogenation of an isopropyl aryl substituent accompanies the b-hydrogen elimination process. As shown in Figure 2, reducing the steric protection enforced by the 2,6-disubstituted aryl ring increases the rate of chain termination. The diethyl substituted compound has a half life of 2 hours; the dimethyl derivative less than 5 minutes. One open question is what is the fate of the iron complex following b-hydrogen elimination of the dimethyl substituted compound? Current efforts are directed toward answering this important question. Perhaps most surprising, introduction of a tert-butyl substituent into the para position of the pyridine ring substantially increases the lifetime of the iron ethyl derivative. A half life of 25 hours was measured at 23 ¼C. As with (iPrPDI)FeCH2CH3, transfer dehydrogenation of an isopropyl aryl substituent is a key component of the b-hydrogen elimination reaction.
Figure 2. Measured half lives for b-hydrogen elimination of bis(imino)pyridine iron ethyl complexes as a function of chelate substituent.
What is the origin of this significant effect? Isotopic labeling studies on the para-tBu substituted compound suggests a similar pathway is operative than the original compound and that a change in mechanism has not occurred. We postulate that dissociation of one of the imine "arms" of the chelate is the first step in the mechanism followed by rotation about the Cipso-Cimine bond. Placing a tert-butyl substituent in the para-position of the pyridine ring likely inhibits imine dissociation as predicted by the Thorpe-Ingold effect. Current efforts in our laboratory are focused on preparing discreet, single component olefin polymerization catalysts based on this bis(imino)pyridine ligand framework. We are particularly interested if the results of the fundamental studies translate on to isolated polymer properties.