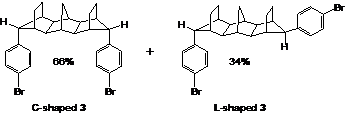Reports: GB7
45830-GB7 Probing Through-Space Charge Migration in Segmented Conducting Polymers
In the last reporting year a total of five undergraduates were working in my research group, all of whom made progress on various aspects of the project. One of our goals has been to synthesize C-shaped monomers 1 and 2 for their eventual incorporation into polymers (Figure 1). We have successfully synthesized C-shaped bisthiophene monomer 1, but cyclic voltammetry has shown that 1 does not undergo electropolymerization under ambient laboratory conditions. Thus, monomer 2 became our next synthetic target since attaching a second thiophene ring would be expected to lower the redox potential enough to allow for electropolymerization. Unfortunately, isolation of C-shaped bisbithiophene monomer 2 continues to elude our synthetic efforts. Our basic strategy for obtaining 1 and 2 has been to attach thiophene rings to dibromide 3 (Figure 2) using Pd-catalyzed coupling methodology, namely Suzuki and Stille coupling reactions.
Figure 1. C-Shaped electroactive monomers 1 and 2.
We have established that dibromide 3 is isolated as a mixture of two diastereomers where approximately 66% is the C-shaped form and 34% is presumed to be the L-shaped form (Figure 2). Because these diastereomers have exceptionally similar physical properties, they have proven to be inseparable by TLC, column chromatography, and recrystallization. Thus, their separation was explored using analytical scale reverse-phase high performance liquid chromatography (HPLC). Several different eluent compositions were surveyed, and optimal separation conditions were found to be 100% acetonitrile as the mobile phase with a 0.60 mL/min flow rate. Under the latter conditions, the C- and L-shaped diastereomers had retention times of 9 and 15 minutes, respectively. Unfortunately due to the price and scarcity of acetonitrile during the recent well-known shortage, we postponed the preparative scale separation of the diastereomers.
Figure 2. Structure of key synthetic intermediate dibromide 3.
Although several variations of Pd-catalyzed coupling reactions were explored, none have produced clean samples of 1 nor any of 2, suggesting that an alternative strategy to putting the thiophene rings on 3 after the C-shape is set should be pursued. We have recently determined that attachment of the second thiophene or bithiophene segment to 3 suffers from serious steric problems during the catalytic cycle. Repeated attempts at synthesizing 2 under different conditions led to isolation of the monoaddition product with a hydrogen atom in place of the bromine on the other benzene ring. Although monomer 1 has been successfully synthesized by a microwave-assisted Suzuki reaction, it was observed that a significant amount of the analogous unwanted monoaddition product was formed along with 1.
One underlying thrust of our recent synthetic work has been to develop greener methods of making all our compounds whenever possible. Acquisition of a CEM microwave reactor in summer 2008 facilitated this objective, and we have since replaced several conventional heating methods with optimized microwave-assisted methods, significantly reducing precious reaction time, solvent, and water/energy consumption.
One of my students had a particular interest in theoretical chemistry, so he undertook a computational investigation of charge transfer in C-shaped monomer 1. A computational method for localizing a +1 charge on one electroactive segment was developed to study charge transfer in the form of polarons. Potential energy surface scans at the B3LYP/6-31G* level indicated that steric interactions between the electroactive segments and the spacer shape the energy landscape more than the direct interaction between electroactive segments as the relative orientation of electroactive segments was altered. The electroactive segments seemed to interact the most with each other at canted conformations, between face-to-face and T-bone orientations. These conformations seemed to avoid most of the negative energetic consequences of the steric interactions and were raised less than 4 kcal/mol in energy. Thus, conformational changes in the electroactive segments are likely when polymers derived from monomers 1 and 2 become oxidized and conductive.
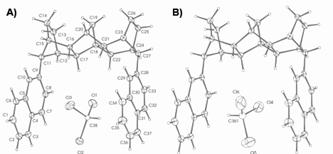
During the pursuit of monomers 1 and 2, we also synthesized structurally related molecule 4 using similar techniques used to obtain dibromide 3 (Figure 3). As can be seen in Figure 3, X-ray quality crystals of 4 were grown, and its X-ray structure was solved. The structure of 4 is roughly C-shaped, which provides a space between the aromatic rings that small molecules can occupy. The solvent of crystallization, CDCl3, was found to occupy the space in two disordered orientations in a 3:2 ratio (Figure 3A and Figure 3B, respectively). These results support our supposition that polymers synthesized from electroactive C-shaped monomers will have well-defined binding pockets that may lead to their potential use as redox-active sensory materials.
Figure 3. X-ray crystal structure of C-shaped 4 with displacement ellipsoids at the 50% probability level.
The Type GB starter grant from the ACS PRF has had a tremendous impact on my academic career, and even more so on the careers of the six undergraduate researchers who worked in my group over the past two years (YR1: 4; YR2: 5, with three students overlapping both reporting years). The research experience they gained in my group has contributed to their being recognized with several competitive national science scholarships and acceptance into prestigious chemistry PhD programs. In the past reporting year, three graduating seniors that worked with me have all matriculated into doctoral programs in chemistry. Of the three students who have been in the group throughout the past two years, one was awarded a 2008 Goldwater Scholarship and an Honorable Mention for his 2009 NSF Graduate Research Fellowship (GRF) application and the second student, a 2008 SUMR recipient, was awarded a 2009 NSF GRF, all while they were still undergraduates. The third student, currently a junior, won a 2009 Goldwater Scholarship where her only research experience at the time of her application was in my group. She was also selected to participate in a highly competitive NSF REU position in summer 2009. This year we presented our research results as four separate posters at two local conferences. I also wrote and submitted two manuscripts, one that is currently published and one that was accepted with minor revisions.