Reports: AC4
48587-AC4 'Radically' Green Approaches To Hydrocarbon Functionalization Via Allyl Transfer
0. Impact
This project supports the efforts of Dr. Liang Chen (postdoc) and Ms. Shraddha Patil (2nd year graduate student). In addition to developing his skills as an organic chemist, this project affords Dr. Chen the opportunity to develop his written and oral communcation skills as well in order to improve his marketability after this project is complete. Dr. Chen is also helping teach Ms. Patil the laboratory techniques and skills needed for this project. Ms Patil is preparing a comprehensive literature review & research proposal as part of the requirements for Ph.D. candidacy at Virginia Tech. It is anticipated that she will "inherit" this project, and that it will provide the foundation for her Ph.D. thesis.
1. Introduction
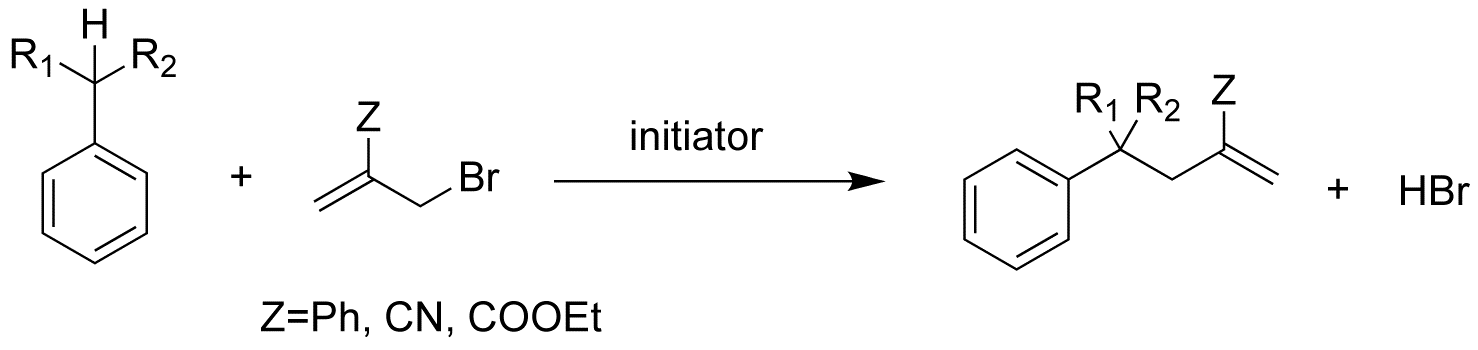
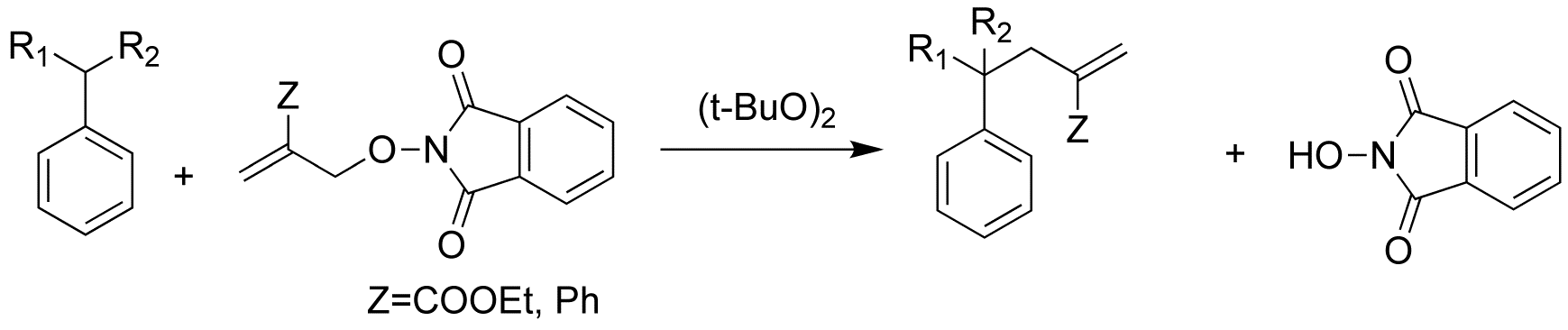
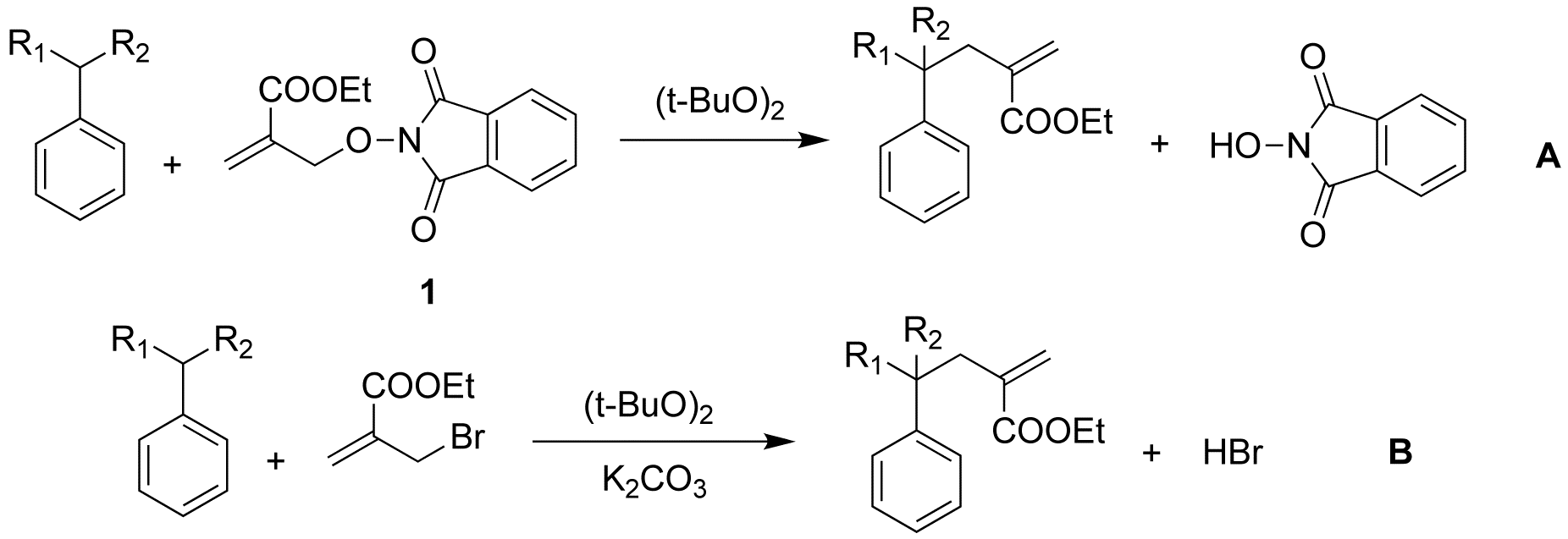
In 1999, we reported a new free radical reaction in which the C-H bond of a hydrocarbon is converted to a C-C bond via transfer of an allyl group (R-H→R-C-C=C). The transformation is accomplished in one step. This reaction uses common thermal initiators, and does not require strongly acid or basic reaction conditions or the use of metals. However, this reaction uses an allyl bromide as a starting material and produces a strong acid (HBr) as by product (Scheme 1).
Scheme 1
In order to make this chemistry "greener", we wanted to remove the bromine from this allyl transfer chemistry. We have now found that phthalimide N-oxyl radical is an excellent replacement for bromine in this allyl transfer process.
2. Experimental and results
2.1 Synthesis of the PINO compounds
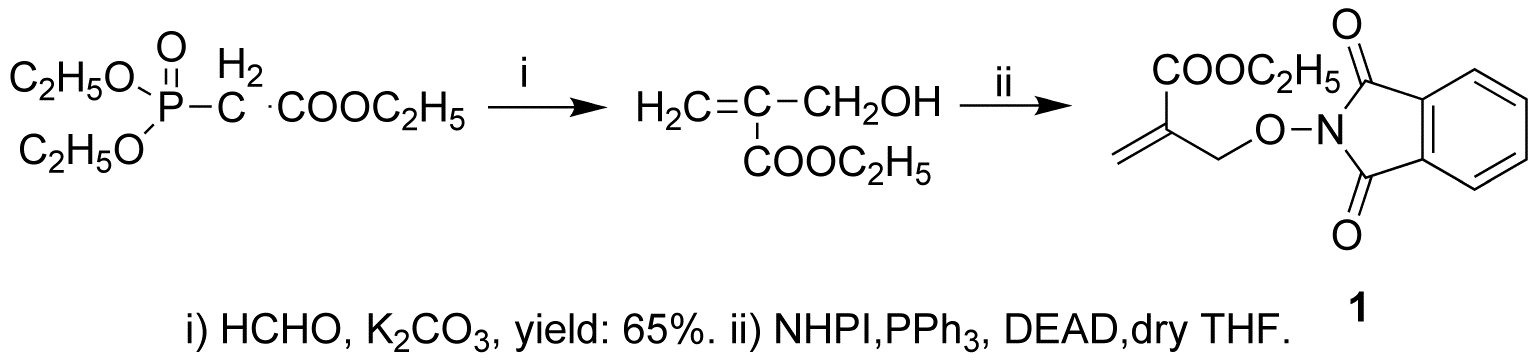
We attmpted to synthesize PINO compound (1) using several published methods. In the first, ethyl a-Hydroxymethylacrylate is reacted with N-hydroxyphthalimide, in the presence of triphenylphosphine and diethyl azodicarboxylate (DEAD), dry THF as solvent, in the room temperature. We tried this reaction several times, but only one time was successful, and the yield was very low. (Scheme 2)
Scheme 2
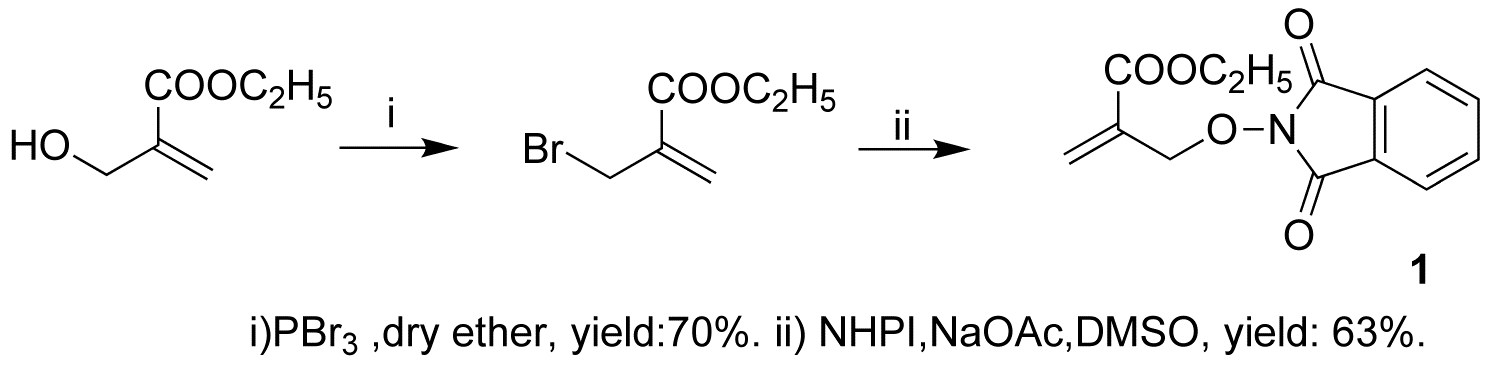
Accordingly, we changed synthetic method. The second method used N-hydroxyphthalimide and Ethyl a-bromomethylacrylate as starting materials, in the presence of sodium acetate, DMSO as solvent, in the room temperature. This reaction works. The yield is about 63%. (Scheme 3)
Scheme 3
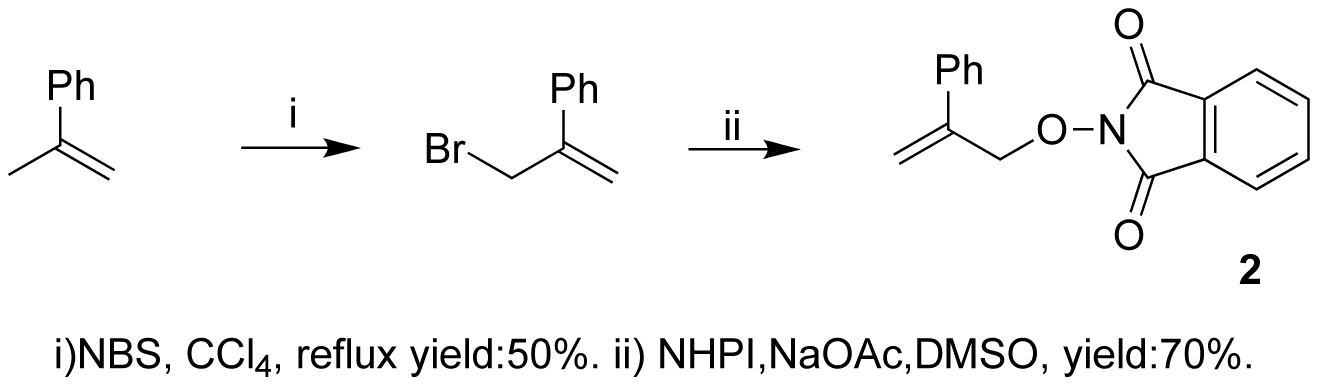
We also synthesized PINO compound (2), using the same method. (Scheme 4)
Scheme 4
2.2 The study of free radical reaction
We studied the free radical reaction based on the PINO compound 1 and 2 (Scheme 5). The results of this study are summarized in the Table 1. In this reaction, N-hydroxyphthalimide that was made in the reaction was precipitated from the reaction system. All the reactions proceeded in excellent yield, save for the reaction of 1 with toluene. Thus far, all attempts at optimization have failed to raise the yield to anything greater than 50%.
Scheme 5
Table 1
|
Entry
|
R1
|
R2
|
Z
|
Time
(h) |
Yield
(%) |
PINO compound left (%)
|
Mass balance (%)
|
||
|
1
|
H
|
H
|
COOEt
|
42
|
48
|
21
|
69
|
||
|
2
|
H
|
CH3
|
COOEt
|
7
|
85
|
7
|
92
|
||
|
3
|
CH3
|
CH3
|
COOEt
|
24
|
91
|
9
|
100
|
||
|
4
|
H
|
H
|
Ph
|
24
|
77
|
23
|
100
|
||
|
5
|
CH3
|
CH3
|
Ph
|
29
|
76
|
22
|
98
|
||
All reactions were performed in neat toluene, ethyl benzene or cumene, using 20 mol % di-tert-butylperoxide as the initiator. The temperature is 120°C.
We also compared the PINO compound to the bromide compound as starting materials (Scheme 6).
The result is showed as followed (table 2). The rate of the reaction with the bromide compound is faster than the rate of the reaction with PINO compound 1. However, the mass balance for the reaction with PINO compound 1 is higher; attempts are now underway to optimize the yield.
Table 2
|
|
Bromide compound |
PINO compound 1 |
||||
|
|
Starting material left |
% yield |
% mass balance |
Starting material left |
% yield |
% mass balance |
|
R1=R2=Ha
|
none
|
35% |
35% |
21% |
48% |
69% |
|
R1=H,R2=CH3b
|
none
|
70% |
70% |
44% |
56% |
100% |
|
R1= R2=CH3b |
41% |
36% |
77% |
75% |
20% |
95% |
a The reaction time is 42 hours, b the reaction time is 3 hours. All reactions were performed in neat toluene, ethyl benzene or cumene, using 20 mol % di-tert-butylperoxide as the initiator. The temperature is 120°C.
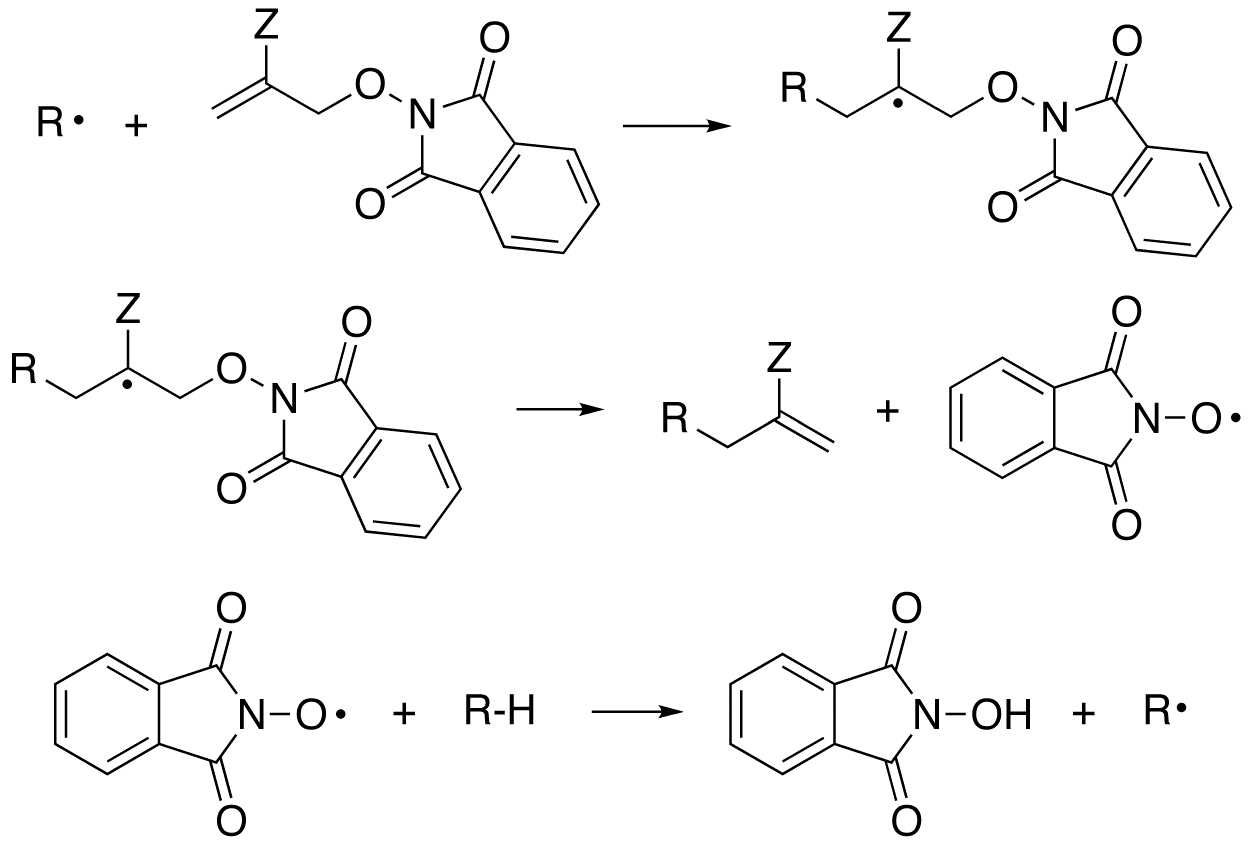
The allyl transfer process with the PINO leaving group proceeds by the mechanism depicted in Scheme 7. Kinetic chain lengths were determined by comparing the rate of product formation with respect to the rate of initiator disappearance. The chain length data are summarized in table 3.
Scheme 7
Table 3
|
Initial chain length
|
||
|
R1 = R2 = H
|
R1 = H, R2 = CH3
|
R1 = R2 = CH3
|
|
266
|
129
|
11.5
|
2.3 Study the free radical reaction using CuCl/PhI(OAc)2 as initiator
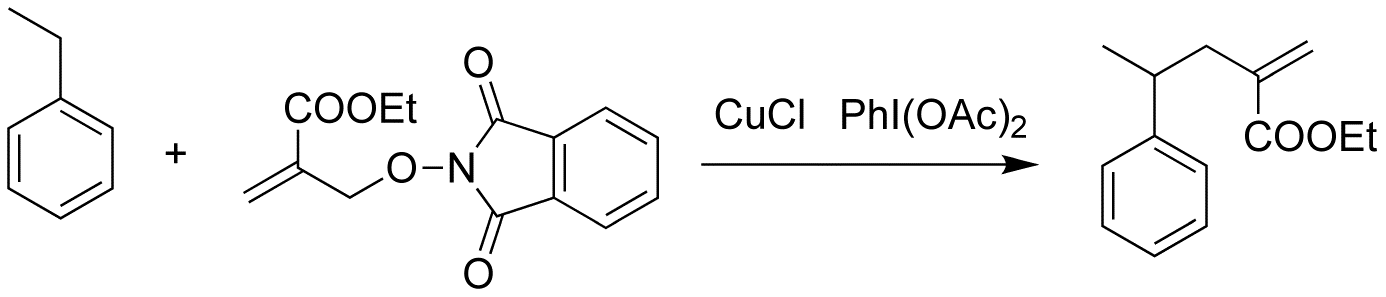
The temperature of this reaction is about 120°C due to use the di-t-buutylperoxide as initiator. So we want to change the intitiator in order to decrease the temperature to room temperature or lower. Recently, Chang's group got the PINO radical from NHPI, using CuCl/PhI(OAc)2 as initiator at the room temperature. So we also attmpted our reaction, using the same initiator.
In our reaction, The CuCl is 10% of PINO compound, PhI(OAc)2 is the same moles of PINO compound, ethyl benzene is also solvent, temperature is room temperature, time is 24 hours. The yield of this reaction is 13%. 79% PINO compound is left. The reaction works, but the yield is terribly low.
4. Future work
We want to synthesize more PINO compounds to continue to study the radical reaction and try to do their free radical reactions and get their chain length data. We also want to find other initiator that can initiate this reaction in the lower temperature. We will specifically looking at low-temperature initiators such are R3B/O2 and tBuON=NOtBu. As discussed in the proposal, laser flash photolysis studies will be initiated in order to better understand the mechanism of this reaction.