Reports: AC1
42853-AC1 Asymmetric Synthesis via Organocopper Chemistry
The aim of this project is to develop efficient asymmetric synthesis strategies involving organocopper chemistry employing protocols that ultimately are amenable to either enantioenriched cuprates or enantioenriched electrophiles (e.g., allylic substrates) or both at the same time. In this period, we have shown that conjugate addition reactions can be effected on 4-pyridones with Grignard reagents and catalytic amounts of copper catalysts or without copper catalysts in the presence of chlorotrimethylsilane (TMSCl). This opens the possibility of using Grignard reagents and chiral copper catalysts for these conjugate addition reactions. We have also found that the same procedures work well for 2-pyrones (eq. 1), providing for the first time effective protocols for conjugate additions to the 2-pyrone framework.
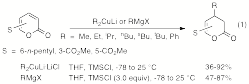
|
While prior work was directed toward controlling the chemo-, regio-, and stereoselectivity in the reactions of organocuprates with 4,5-epoxy-2-enoates and 4-halo-5-acetoxy-2-hexenoates, we have now examined vinyl oxirane 1 in order to probe the generality of the method. Here regio- and diastereoselectivity proved more difficult to control, but optimal conditions were found that gave very good to excellent selectivities (eq. 2) for alkyl transferable ligands. The method generates vicinal stereogenic centers and an allyl silyl ether (i.e., 3) providing functionality for subsequent synthetic elaboration. Enantioenriched vinyl oxirane 1 is readily available via Sharpless asymmetric epoxidation providing an efficient synthetic route to enantioenriched synthons containing vicinal dialkyl substituted stereogenic centers.
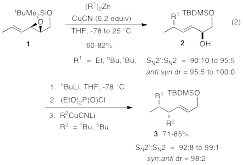
|
We have also uncovered an interesting reaction of tri-organozincates, which regioselectively open the 4,5-epoxide of ethyl sorbate (4) in a regioselective SN2 fashion followed by a reduction of the carbon-carbon double bond to afford 5. While the reaction works well for Me, Et, and n-Bu ligands, complicated product distributions are obtained for aryl- and heteroarylzincates. The method provides for a rapid synthesis of trans 5,6-dialkyl substituted delta-lactones 6.
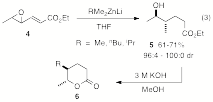
|
Support for this project has enabled us to develop methods for tandem and sequential organocopper mediated bis-allylic substitution reactions for generating contiguous stereogenic centers in a stereocontrolled fashion, and for developing copper catalyzed conjugate addition reactions to 4-pyridones and 2-pyrones not previously observed. The funding was essential for us to maintain an active program in synthetic methodology development. This support by ACS-PRF has enabled one student to complete his Ph.D. work and another to make substantial progress toward a degree.




