Reports: AC10
45283-AC10 Porous and Biphasic Materials through Solid State Reactions
In our final report on research supported by the ACS PRF funding, we would like to summarize our work over the entire course of the grant, and provide some insight to the implications and continuing projects that are its direct consequences. We have investigated routes to porous and functional inorganic materials and bifunctional composites via solid-solid and solid-vapor reactions. These spontaneous, template-free processes are versatile, rapid routes which may be utilized in many fields, including filtration, catalysis, magnetic data storage, charge trapping, etc.
Two recent review articles have resulted from our work supported by ACS PRF. Ram Seshadri served as Guest Editor for the August 2009 issue of the Materials Research Society Bulletin, and contributed an article on hard materials with tunable porosity. The cover (Figure 1a) displays micrographs from our ACS PRF publications. Additionally, an invited review article on template-free porous materials will soon appear in the Journal of Materials Chemistry.
- Vapor-phase leaching
Our production of hard, template-free porous materials has centered around vapor phase leaching. Heating oxides in a flowing stream of 5% H2/N2 provides a reducing environment that allows conversion between transition metal oxides and metals. Because H2 is a sufficiently strong reducing agent, this transformation occurs at relatively low temperatures and pores are often nano-sized. For example, reduction of Mn3O4 to MnO at 450°C is accompanied by significant volume loss, manifested in an open network of 20-100 nm pores. The oxygen sublattice is conserved during the process. Since the crystalline orientation is maintained, pores have aligned, square edges over the extent of each grain. We can close the induced micropore network by reoxidation.
In ternary oxides such as NiMn2O4, we demonstrated facile conversion of a single-phase material into an intimately mixed, highly-interfacial composite. At 725°C, the the reduction reaction proceeds in two distinct steps as:
NiMn2O4 + H2 → 3Ni0.33Mn0.67O + H2O(↑) → Ni + 2MnO + H2O(↑)
The resulting composite (Figure 1b) has 25 nm Ni nanoparticles completely encased inside the porous MnO matrix. We observe magnetic exchange bias due to intimate contact between ferromagnetic Ni and antiferromagnetic MnO. Redox processes allow egress and ingress of metal nanoparticles, so we can tailor this process to produce catalytic metals supported on oxides.
- Thin film applications
Precision and ordering of the porosity in vapor-phase leached materials is especially apparent in our single-crystalline MnO thin films. ZnMn2O4 was deposited hydrothermally on (100) and (111) MgAl2O4 substrates. Reduction of these epitaxial films in 5% H2/N2 at 450-650°C removes Zn and O, resulting in MnO films with an open network of micropores. On (100) substrates, all pore faces are aligned parallel or perpendicular to the substrate. On (111), the pore edges are rotated by 54.75° with respect to the substrate. Pore sizes are 50-100 nm on (100), and 30-50 nm on (111). The epitaxial agreement between MgAl2O4 and ZnMn2O4 permits tailoring of the pore orientation and size by choosing the plane of growth. Because the reduction occurs at temperatures well below the melting point of MnO (1840°C), pore channels remain fully open during firing, and the microstructure does not change with film thickness.
- Advanced local structure and magnetic characterization of heterogeneous oxides
At its core, our research has focused on oxides that are disordered on the nanoscale. One especially useful extension of this approach is to examine "single-phase" materials with tools historically used to characterize liquids, glasses, and other heterogeneous materials. We used high-resolution total scattering and reverse Monte Carlo (RMC) modeling to investigate the local structure of CuMn2O4. We expect d9 Cu2+ to be Jahn-Teller active on the tetrahedral site, but not Mn2+/3+. We extracted bond angle distributions from the RMC supercell (Figure 1c) and found that the distribution of BO-Cu-O(θ) is fit much better by two Gaussians than by one, indicating distortion. The single-peak fit for BO-Mn-O(θ), on the other hand, exhibits no distortion. This is of great importance because the quintessential tool of crystallographers, Bragg Rietveld refinement, does not allow specificity between two different cations on the same site.
Comparison of our bond valence sums with x-ray photoelectron spectroscopy (XPS) results, showed that the presence of Cu3+ in the RMC simulations was in fact corroborated by an XPS peak previously attributed to tetrahedral Cu2+.
We also examined the heterogeneity of a the ZnxMn3-xO4 solid solution with x ≤ 1, which shows a strongly shifted exchange-biased magnetic hysteresis loops for x > 0.5 (Figure 1d). Magnetically, this material behaves like two distinct phases: an antiferromagnet and ferromagnet. However, diffraction patterns reveal it to be single-phase. Though a comprehensive suite of magnetic measurements we found that the intrinsic exchange bias is a result of nanoscale heterogeneity, but not the random atomic mixing seen in glassy magnetic materials.
- Personal Impact
Since receiving support from the PRF, Ram Seshadri has received tenure in the UCSB Materials Department, been named Assistant Director of the UCSB Materials Research Laboratory, and become Associate Editor of the Journal of Materials Chemistry. Eric S. Toberer has earned his PhD in Materials. He is currently in a postdoctoral position at Caltech and was awarded a Beckman Fellowship. Graduate student Daniel P. Shoemaker entered the group in Fall 2006, advanced to candidacy in Fall 2008, was awarded a fellowship from the UCSB-Los Alamos Institute for Multiscale Materials Studies, and plans to defend his thesis in Fall 2010.
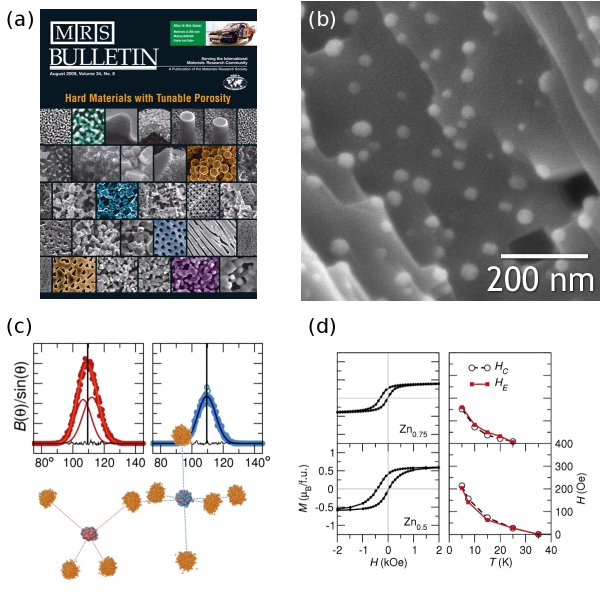
Figure 1. (a) August 2009 MRS Bulletin cover focusing on our work. (b) Ni-MnO nanocomposite produced by vapor-phase leaching. (c) Tetrahedral and octahedral environments in CuMn2O4 from RMC modeling of total scattering, and the respective bond angles for tetrahedral Cu (red) and Mn (blue). (d) Shifted hysteresis loops, coercive field HC and exchange bias field HE of the nanoscale-heterogeneous oxide solid solution Zn0.75Mn2.25O4 (top) and Zn0.5Mn2.5O4 (bottom).




