Reports: GB5
45955-GB5 Bonding and Electronic Structure of Reconstructed Surface Alloys
1
6
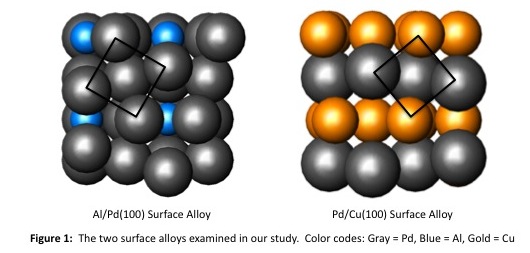
Using the funding provided by this grant, we have investigated the electronic structures of Al/Pd(100) and Pd/Cu(100), two surface alloy systems that undergo a similar "clock"-type reconstruction (Figure 1). These studies were carried out using the Fenske-Hall band structure method, which is an approximate Hartree-Fock type method. In our last report, we described how the reconstruction of the Al/Pd(100) surface alloy optimizes both the Pd-Pd bonding within the surface layer and the Pd-Al bonding between the first and second layers of atoms. In this report, we shift our focus to the Pd/Cu(100) surface alloy.
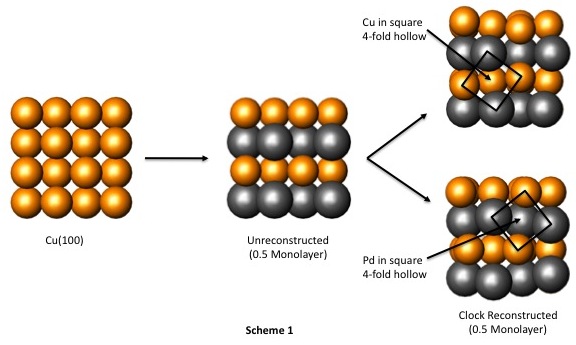
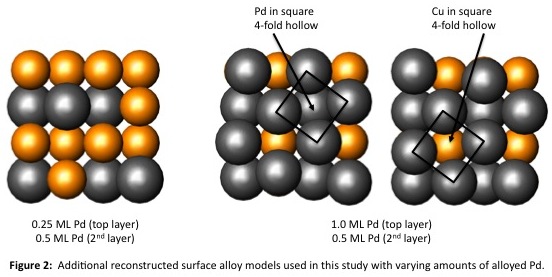
To analyze the electronic structure of the Pd/Cu(100) surface alloy, we employed a stepwise theoretical analysis similar to our Al/Pd(100) studies. We began by examining models of bulk Cu, a clean Cu(100) surface, a hypothetical unreconstructed Pd/Cu(100) surface alloy, and two clock reconstructed surface alloys with Pd coverages that correspond to 0.50 monolayer in both the first and second layers of atoms (Scheme 1). It should be noted that these two reconstructed surfaces differ only in the location of the "square" fourfold hollow sites created by the clock reconstruction: in the upper reconstructed model of Scheme 1, the square fourfold hollow surrounds a second-layer Cu atom, whereas in the lower model the square fourfold hollow surrounds a Pd atom. We also examined models of the reconstructed Pd/Cu(100) alloy with different amounts of Pd coverage in the first two atomic layers, based on actual structural data reported by Pope and coworkers (Pope, et al., Surf. Sci. 337 (1995) 79-91) (Figure 2). Our goal in studying these particular models was to correlate features of the electronic structures of these systems with existing knowledge of their physical structures; however, we have encountered difficulties in our analyses of Pd/Cu(100) system that we did not observe in the Al/Pd(100) system.
As noted above, our investigation of the Al/Pd(100) system revealed that when the surface alloy reconstructs, bonds within the surface plane and between surface- and second-layer atoms are strengthened. These observations were supported by calculated overlap populations. If we think of a chemical bond as the overlap between the orbitals on neighboring atoms, we can relate the strength of the bond to the extent of the overlap of the orbitals. The overlap population reflects this, as it is an indication of how many electrons reside in bonding orbitals within a molecule. Unfortunately (for our studies), bond overlap populations only apply to covalent bond character, and do not provide any insight as to the strength of a bond that is more metallic in nature. In the case of the Al/Pd(100) alloy, the bonding can be described as slightly polar covalent, despite the fact that all of the atoms in the system are metallic. Using the Pauling Scale, the difference in electronegativities between Al (1.61) and Pd (2.20) is 0.59, which is just large enough to be considered as slightly polar. In the Pd/Cu(100) surface alloy, however, the difference in electronegativities is only 0.30. As a result, the bonding is considerably more delocalized in the Pd/Cu(100) system. Because of this, we were unable to determine any significant differences in the bonding between any of our model surfaces.
Although our analyses using the Fenske-Hall method have reached their limit with the Pd/Cu(100) system, we would like to continue our study by comparing the total energies of the different reconstructed models using density functional theory (DFT). Since DFT does not rely on wavefunctions like the FH method, we hope to use the results of our calculations to predict the most likely structures for the Pd/Cu(100) surface alloy at different amounts of Pd coverage. Thus, we ask that the PRF consider granting us a one-year extension to accomplish this part of the project.