Reports: GB3
47921-GB3 Molecular Models for Bi/Mo Oxidation Catalysts
Bismuth has an extremely diverse chemistry with applications in materials science, medicine, organic synthesis and catalysis, among others. With respect to the field of catalysis, arguably the single-most important process is the selective oxidation/ammoxidation of propene to make acrolein (eq 1) and acrylonitrile over a multi-component catalyst based on Bi2O3×MoO3 known more commonly as the SOHIO process. Both acrolein and acrylonitrile are currently used in industry on large scales. It was recently reported that, in the year 2000, the worldwide demand for acrylonitrile alone was approximately 5,000,000 tons.
Despite the commercial success of the SOHIO process and considerable subsequent research, the exact mechanism by which the catalyst is able to facilitate the observed chemical transformations remains unclear. It is possible that additional insight into the mechanism of functioning of the SOHIO oxidation catalyst could be gained through the investigation of molecular complexes whose geometry and bonding arrangements mimic what is seen in the bulk material. Unfortunately, molecular bi/Mo complexes are scarce, with species that contain structural features associated with the bulk SOHIO oxidation catalyst (including Bi-OH, Bi-O-Bi, Mo-O-Bi and Mo=O moieties) being particularly elusive.
In an attempt to circumvent some of the difficulties associated with the synthesis of discrete molecular coordination complexes that contain bismuth, our group has explored an alternate bottom-up synthetic approach. Our strategy relies on the initial formation of a structurally robust polynuclear bismuth core, which can be subsequently functionalized with transition metal fragments and produce the desired heterobimetallic species.
The polynuclear bismuth core that we have selected for this project is the recently discovered cationic nonanuclear oxo hydroxo complex Bi9(m3-O)8(m3-OH)6(ClO4)5 ([1]5+).19 or the tetraethyleneglycol functionalized derivative Bi9(m3-O)8(OCH2CH2OCH2CH2OCH2CH2OCH2CH2O)3(ClO4)5. ([1-EO4]5+) These complexes were selected for study due to the fact that:
(1) they can be easily produced in high yields
(2) the bismuth-oxygen bond angles and distances strongly mimic what is seen in the solid state structure of both a and b-Bi2O3,
(3) The bismuth-oxygen framework in both complexes is physically large enough to potentially accommodate multiple transition metal fragments, thereby allowing for the production of a variety of heterobimetallic coordination complexes for subsequent study and comparison.
(4) [1-EO4]5+ is readily soluble in polar organic solvents and can be recrystallized without apparent structural change or decomposition
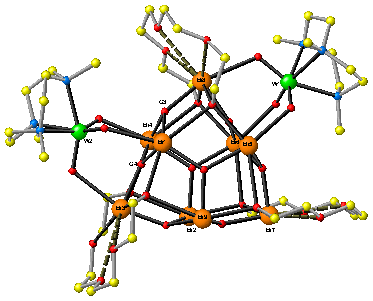
We have found that [1-EO4]5+ reacts smoothly with two or three equivalents of (TMTACN)MO3 (TMTACN = 1,3,5 trimethyl 1,3,5 triazacyclononane; M = Mo, W) to produce the undecanuclear heterobimetallic species ((TMTACN)MoO3)2(Bi9(O)8(EO4)3)(ClO4)5∙4CH3CN [2]5+(80% isolated yields) and ((TMTACN)WO3)2(Bi9(O)8(EO4)3)(ClO4)5∙4CH3CN (85% isolated yields) [3]5+ (Fig. 1), as well as the dodecanuclear complex {(TMTACN)WO3}3∙(Bi9(m3-O)8(m3-OR)6)(ClO4)5 [4]5+
Fig. 1: Detail of the solid-state structure of the cationic core of [3]5+.
Structural analysis of the complexes reveals that there is relatively little perturbation in the structure of [1-EO4]5+ in the course of adduct formation with the (TMTACN)MO3 fragments. In all cases, the trioxo transition metal fragment is associated with the bismuth core via a triply bridging m3:h1,h1,h1 coordination mode. The bridging Mo∙∙∙O∙∙∙Bi bond distances are asymmetric and best described as a Mo=O∙∙∙Bi type dative interaction.
The preferential orientation of the (TMTACN)MO3 transition metal fragments along the C2 symmetric faces of the bismuth core appears to be driven by steric interactions between the tetraethyleneglycolato ligands associated the bismuth oxo-alkoxo framework and the methyl groups on the transition metal fragment.
We have been able to confirm that the (TMTACN)MO3 transition metal fragments remain associated with the cationic bismuth core in solution via solution state n.m.r. and electrospray mass spectrometry experiments. The solution-state n.m.r. spectra of [2]5+, [3]5+ are consistent with the solid-state structures and suggest that the compounds that we have been able to isolate and characterize to be the products that are preferentially formed in the course of these reactions. We are currently in the process of finishing the complete characterization of these three complexes and look forward to publishing the results of study in the near future.
The apparent stability of the heterobimetallic species [2]5+ and [3]5+ is important and suggests that that the cationic nonabismuth complex [1]5+ (or alkoxo derivatives thereof) can act as a viable scaffold for the construction of compositionally complex heterobimetallic coordination compounds. The ability of the bismuth core to accommodate multiple transition metal fragments without itself undergoing significant structural changes suggests that the synthetic strategy that we have developed in this work may offer an unprecedented opportunity to design targeted syntheses for high nuclearity heterobimetallic bismuth based coordination complexes.
In the coming year, we will use the results of our initial studies to (1) develop methods to strengthen the interaction between the bismuth core and transition metal fragments and (2) explore the association of transition metal fragments that bear potentially reactive sites including alkoxo, amido or terminal Mo=O functional groups to [1-EO4]5+.
We acknowledge the support of the Petroleum Research Fund in the development of this project. Financial resources provided as part of PRF grant# 47921-GB3 were instrumental in the realizing the successes described in the narrative above. Specifically, funding from the PRF allowed for the purchase and installation of a glovebox in the PI's lab, supported student worker salaries for the duration of the academic year and summer session and provided access to the single crystal x-ray diffraction and high resolution n.m.r. spectroscopy experiments necessary to elucidate the structure and chemistry of the complexes that we are exploring.