Reports: B3
44891-B3 Preparation, Characterization, Film Fabrication, and Photoluminescence of Metal-Organic Networks of Copper(I) Cyanide
Over the last year the research focus has been on the reactions of copper(I) cyanide with liquid and vapor-phase amines and imines (L). The goal of this work is the development of luminescent detection methods for volatile nucleophiles in the environment. Intensely luminescent (CuCN)Ln (n = 0.5, 0.75, 0.8, 1, 1.25, 1.5, 2) complexes result from exposure of CuCN to L in the liquid or vapor state. The ligands used in the study were Py = pyridine, 2MePy = 2-methylpyridine, 3MePy, 4MePy, 2EtPy = 2-ethylpyridine, 3EtPy, 4EtPy, 3BrPy = 3-bromopyridine, 4tBuPy = 4-t-butylpyridine, 26Lut = 2,6-lutidene, 246Coll = 2,4,6-collidine, Quin = quinoline, 1MeIm = 1-methylimidazole, NEt3, NHEt2, NHiPr2, Pipd = piperidine, MePipd = N-methylpiperidine, EtPipd = N-ethylpiperidine, MePyrrolid = N-methylpyrrolidine, Morph = morpholine, MeMorph = N-methylmorpholine, and NMe2Cy = N,N-dimethylcyclohexylamine.
The methods used for CuCN-L formation are outlined in Figure 1. Authentic CuCN-L samples were prepared by heating CuCN in neat liquid L. In many cases, especially for aromatic imine ligands, initially formed L-rich phases, such as (CuCN)(2MePy), could be converted to L-poor phases, such as (CuCN)2(2MePy)3, via vacuum treatment. Chemisorbed surface adducts were formed by adding L as a liquid or vapor to CuCN (see below).
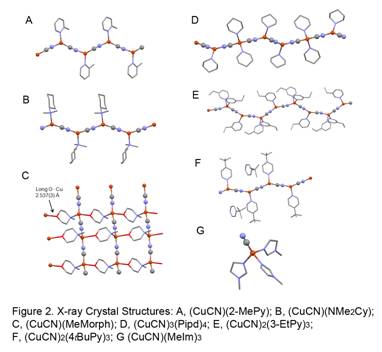
A total of seven new X-ray crystal structures of authentic samples were determined (Figure 2), reflecting a wide variety of coordination arrangements. For example, while (CuCN)(2MePy) and (CuCN)(NMe2Cy) were simple decorated chains with 3-coordinate copper, (CuCN)(MeMorph) showed a long Cu O interaction, producing a sheet structure. (CuCN)3(Pipd)4 featured one 4-coordinate copper for every two 3-coordinate copper atoms. (CuCN)2(3EtPy)3 showed alternating 3- and 4-coordinate copper centers, while (CuCN)2(4tBuPy)3 had only 3-coordinate coppers plus a half molecule of solvent 4tBuPy. Finally and quite surprisingly, (CuCN)(MeIm)3 was found to exist as a monomer.
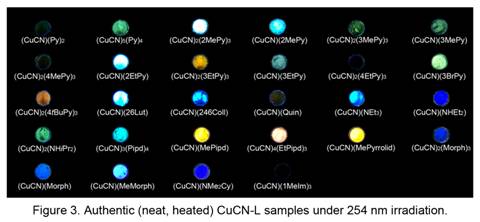
Luminescence photos of the various authentic amine and imine complexes of CuCN are shown in Figure 3. The colors are in agreement with those seen in the liquid drop and vapor diffusion samples. The luminescence behavior of the authentic CuCN-L complexes has been investigated collaboratively by the P.I. with Prof. Howard Patterson (University of Maine, variable temperature spectroscopy) and Prof. Craig Bayse (Old Dominion University, computational analysis). The major findings are:
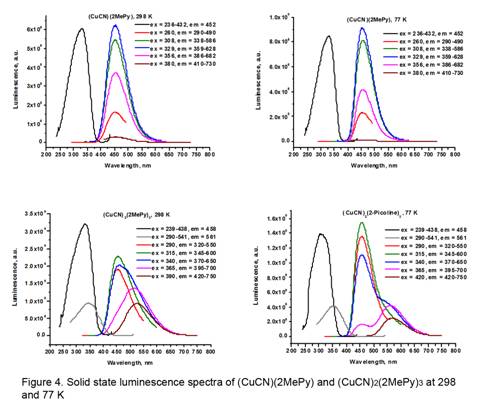
1) There are two basic trends in the luminescence results which are illustrated for (CuCN)(2MePy) and (CuCN)2(2MePy)3 in Figure 4. Many of the CuCN-L products show a single short wavelength (high energy, HE) band around 450 nm, producing a blue emission color. Other products show a long wavelength (low energy, LE) band in addition to the HE band. The LE band is thermochromic, decreasing in intensity at low temperature.
2) Density Field Theory (DFT) calculations suggest that excitation in CuCN involves pi MOs combining metal (3d → 4p) and cyano (pi → pi*) contributions. Excitation is thus an admixture of metal-centered (MC) and metal-to-ligand charge transfer (MLCT) transitions. Hence, the oft-made distinction between MC and MLCT is probably not meaningful for CuCN networks.
3) DFT results suggest that the triplet state is lowered in energy via bending of the CuCN at copper. Since this effect opens a potential coordination site, it may ease reaction with incoming nucleophiles, facilitating exciplex formation. Conversely, bent CuCN-amine complexes should be expected show lower energy emission than does CuCN, as is experimentally observed.
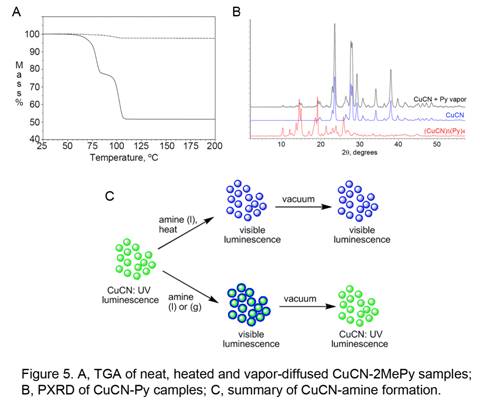
X-ray powder diffraction (PXRD) and thermogravimetric analysis (TGA) have been used to determine the difference between the liquid drop or vapor diffusion (chemisorbed) samples and the authentic CuCN-L products from neat, heated samples (see Figure 1). As shown in Figure 5A, an authentic product shows a large mass decrease due to ligand loss, while a chemisorbed sample shows only a trace of ligand loss. Figure 5B demonstrates that a chemisorbed sample is primarily composed of CuCN plus a small amount of (CuCN)Ln phase. The current understanding of the CuCN-L system is summed up in Figure 5C. During the neat, heated reaction (upper path in Figure 5C), CuCN is fully converted to a pure authentic (CuCN)Ln phase (n = 0.5, 0.75, 0.8, 1, 1.25, 1.5, 2) as indicated by PXRD. The value of n is reproducible and can be determined from TGA or elemental analysis data. Most of the authentic products are highly luminescent in the visible region. Application of vacuum can convert a more L-rich (CuCN)Ln phase to a less L-rich phase, but does not regenerate CuCN. On the other hand, exposure of CuCN to L liquid or vapor at ambient temperature (lower path in Figure 5C) produces a chemisorbed adduct. The latter probably consists of a surface coating of (CuCN)Ln phase on CuCN particles, as suggested by the TGA (Figure 5A) and PXRD (Figure 5B) results. Although the chemisorbed adducts show identical luminescence color to the analogous authentic (CuCN)Ln phases, they revert to CuCN upon application of vacuum, with loss of visible luminescence.