Reports: G10
47110-G10 Control of Methane Hydrate Formation at the Molecular Level
It is believed that the surface of liquid water is crucial for the formation of methane hydrates since methane concentrations are high at the interface so that the interfacial region would provide the environment for hydrate initiation. Several experimental studies of the interfacial hydrate formation demonstrated an initial film thickness of about 10 mm for various hydrates on the water side of the interface[1]. However, little is known about the molecular-level understanding of the interface under hydrate-forming conditions due to a lack of methods capable of providing high-resolution structural information in compressed gases. In this report, we show that the hydrate formation on the molecular scale has been triggered within 1 min in the hydrate stable region.
 We focused on a stationary interface
separating methane and liquid water by
combining a neutron reflectivity (NR) technique, which is sensitive to interfacial
structures at the angstrom scale, and laser reflectivity (LR), which provides with micron-scale
structure information.
In order
to mimic environmental
conditions of methane hydrate, we have built a high-pressure cell. Deuterated
methane (CD4, 99.9%) and deuterated oxide
(D2O, 99.9%) were chosen to
enhance neutron scattering contrast as well as to reduce incoherent neutron
scattering generated by H atoms.
The phase diagram
for the D2O/CD4 system was obtained by shifting the
equilibrium temperature of the phase diagram for H2O/CH4
by 2.5ºC due to the deuterium isotope effect[2]. D2O of 10 mL was
loaded on a water trough and methane was loaded with
given pressures.
We focused on a stationary interface
separating methane and liquid water by
combining a neutron reflectivity (NR) technique, which is sensitive to interfacial
structures at the angstrom scale, and laser reflectivity (LR), which provides with micron-scale
structure information.
In order
to mimic environmental
conditions of methane hydrate, we have built a high-pressure cell. Deuterated
methane (CD4, 99.9%) and deuterated oxide
(D2O, 99.9%) were chosen to
enhance neutron scattering contrast as well as to reduce incoherent neutron
scattering generated by H atoms.
The phase diagram
for the D2O/CD4 system was obtained by shifting the
equilibrium temperature of the phase diagram for H2O/CH4
by 2.5ºC due to the deuterium isotope effect[2]. D2O of 10 mL was
loaded on a water trough and methane was loaded with
given pressures.
NR measurements were performed at the NIST Center for Neutron Research NG-7 reflectometry. The temperature of 6.5°C was chosen to prevent the formation of ice and the condensation of water at the sapphire windows. The critical pressure (Pc), above which the stably hydrate forms, is predicted to be 3.7MPa at 6.5°C. During the experiments, the sample was not stirred to favour hydrate formation. A position sensitive detector was used for the NR experiments to characterize the specular and diffuse components simultaneously. LR experiments were conducted with a He-Ne laser beam. The incident and exist angles of the laser were fixed to 15° and an aperture was set in front of a photodiode detector to capture the only specular component of the reflected beam.
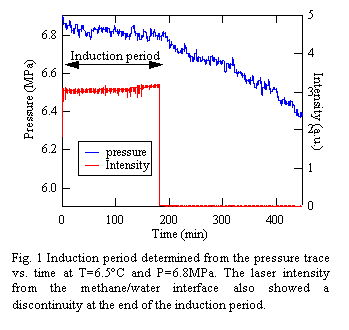
Before reflectivity experiments, the induction period was determined from the pressure trace at the hydrate forming conditions. Fig.1 shows the pressure change as a function of time at T=6.5°C and P=6.9MPa. From the figure we can see the pressure drop at 183 min.
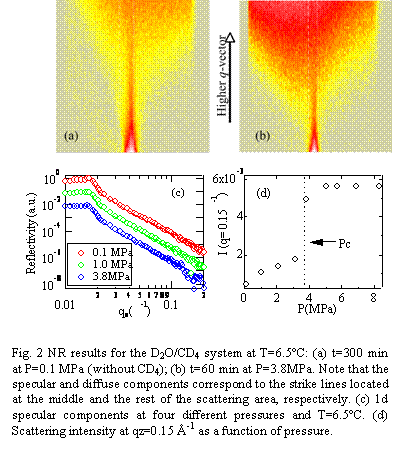 Figure 2 (a) and (b) show
representative two-dimensional NR data at T=6.5°C: (a) P=0.1 MPa and (b)
P=3.8MPa (under the hydrate condition). Note that no significant time evolution of
the scattering patterns was observed for 8 h at the pressures used in this
study (0.1≤P≤8.3MPa).
From the figure we can see that the specular component became weaker, while
diffuse scattering increased considerably. In order to quantitatively
characterize the surface structure, the 2d scattering patterns were decomposed
into the specular and diffuse components. Fig. 2(c) shows the pressure
dependence of the one-dimensional specular components as a function of the wave
vector transfer normal to the surface. The solid lines in Fig. 2(c) correspond
to the calculated reflectivities using the density model based on the Parratt's
algorism. The best-fitted results gave us the root mean square (rms) roughness
(s)
of 10 ± 3Å at the interface at P>Pc, while the svalue
was 3Å in air and slightly increased to 5 Å just below Pc. This surface
roughening at P>Pc was also confirmed by the diffuse scattering intensity.
As shown in Fig. 2(d), the diffuse scattering intensity at qz=0.15 Å-1
showed a discontinuous increase at P=3.8MPa. Hence, the NR results give us the
clear evidence that the interface undergoes the transition from a liquid-like
smooth surface to a more roughened surface at the nanometer length scale as
soon as the water phase contacts with methane gas in the hydrate stable region.
Figure 2 (a) and (b) show
representative two-dimensional NR data at T=6.5°C: (a) P=0.1 MPa and (b)
P=3.8MPa (under the hydrate condition). Note that no significant time evolution of
the scattering patterns was observed for 8 h at the pressures used in this
study (0.1≤P≤8.3MPa).
From the figure we can see that the specular component became weaker, while
diffuse scattering increased considerably. In order to quantitatively
characterize the surface structure, the 2d scattering patterns were decomposed
into the specular and diffuse components. Fig. 2(c) shows the pressure
dependence of the one-dimensional specular components as a function of the wave
vector transfer normal to the surface. The solid lines in Fig. 2(c) correspond
to the calculated reflectivities using the density model based on the Parratt's
algorism. The best-fitted results gave us the root mean square (rms) roughness
(s)
of 10 ± 3Å at the interface at P>Pc, while the svalue
was 3Å in air and slightly increased to 5 Å just below Pc. This surface
roughening at P>Pc was also confirmed by the diffuse scattering intensity.
As shown in Fig. 2(d), the diffuse scattering intensity at qz=0.15 Å-1
showed a discontinuous increase at P=3.8MPa. Hence, the NR results give us the
clear evidence that the interface undergoes the transition from a liquid-like
smooth surface to a more roughened surface at the nanometer length scale as
soon as the water phase contacts with methane gas in the hydrate stable region.
In order to further investigate the surface roughening in the early stage of the induction period, the detector position was fixed to qz=0.15 Å-1, and the time dependence of the intensity was recorded at a 1 min interval. As a result, we found that the diffuse scattering reached to the equilibrium intensity shown in Fig.2 (d) within 1 min regardless of the magnitude of the driving force and no significant time evolution in the intensity was observed during the induction period. This supports the proposed mechanism of the hydrate formation1: oligomeric precursors are formed, but flickering until the end of the induction period when such structures will be turn into polymeric hydrates. In fact, as shown in Fig. 1, the LR experiments at T=6.5°C and P=6.9MPa clearly showed a discontinuity in the specular intensity at 183 min, indicating the formation of a micron-scale rough surface structure. Interestingly, this time is in good agreement with the induction period of time for macroscopic hydrates (Fig.1). Consequently, the above results led to the conclusion that the nucleation of the metastable hydrate structures on the molecular scale is proceeded at the interface during the induction period.
1. Sloan, E.D., 1998. Clathrate Hydrates of Natural gases, 2nd Ed., Marcel Dekker, New York.
2. Chun, M.-K.; Yoon, J.-H.; Lee, H. J. J. Chem. Eng. Data 1996, 41, 1114.




