Reports: AC5
47369-AC5 Thermally Stable, Sintering Resistant Catalysts
A unique way of growing and controlling the elemental composition, size and density of bimetallic Pd-Au nano clusters has been developed. These were our building blocks to assess chemical reactivity towards conversion of acetylene to either ethylene or benzene, both are industrially important reactions. We have made the initial steps towards studying the effect of surface structural defects on the thermal stability of our working catalyst.
The main impact of this research has been to focus my research towards more model-catalysis oriented studies with potential applications in energy related topics. The main body of this research has been performed by a graduate student, Mr. Elad Gross. The impact of this experience on Elad has been in gaining new and original knowledge. He is now well prepared for his next step as a Post-Doc in a leading laboratory within a few months, where he plans to integrate this knowledge into complementing but different field of science and technology.
1. TEM characterization of bimetallic Au-Pd nano-clusters (HUJI)
The buffer layer assisted growth (BLAG) method for growing and depositing metallic nano-clusters has been developed to grow alloy bimetallic (Pd-Au) clusters, based on the multiple cycle BLAG procedure.
Double cycle BLAG procedure performed on amorphous carbon substrate (a-C), however, the second cycle has been used to deposit a different element.
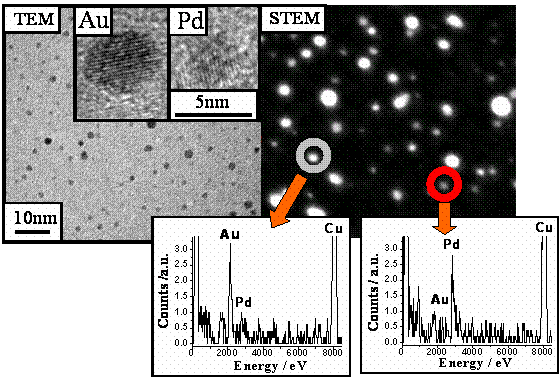
Figure 1 reveals low density (obtained when up to 40-100ML H2O are used as buffer and low metal deposition rate) of Au and Pd deposited via two stage BLAG procedure. The darker (brighter) clusters are gold. It is clear that clusters from both elements grow as crystallites, as determined by Fourier analysis of the periodic layers observed in each of the clusters. The image is obtained ex-situ after raising the sample to room temperature in atmosphere.
Figure 1: HR-TEM image of gold and palladium nano-clusters grown via BLAG following deposition of 0.5Å of each of the elements on 40ML layer of amorphous solid water (ASW).
Energy dispersive X-ray (EDX) analysis confirms the elemental assignment of each of the metallic clusters.
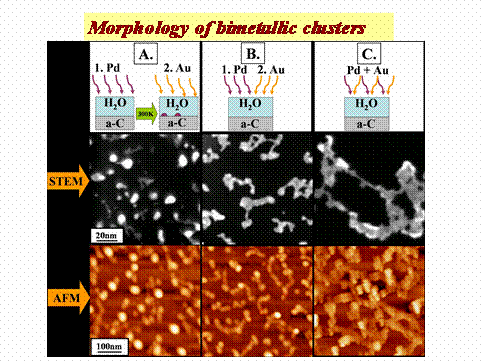
Clusters morphology obtained at higher elemental doses are shown in Fig. 2, where scanning-TEM and AFM images are compared.
Figure 2: Scanning TEM (STEM) and AFM images of 2Å Pd and 2Å Au bimetallic clusters grown via BLAG employing different pathways of BLAG deposition.
2. Reactivity tests: Acetylene conversion to ethylene and trimerization to benzene on Au-Pd bimetallic clusters
Chemical reactivity tests on top of our model supported Au-Pd bimetallic catalysts were performed. Reactivity tests were chosen with acetylene as parent molecule, monitoring ethylene formation and trimerization to form benzene. Both are very well studied on top of extended Pd single crystal surfaces.
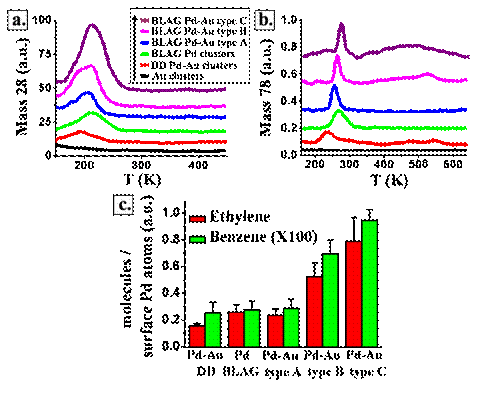
Figure 3: TPR of ethylene (mass28) (a) and benzene (mass 78) (b) collected following adsorption of 2L C2H2 on the different clusters (explained in Fig. 1) at 120K. In the former (type A clusters) separate metallic clusters are formed while in the latter (Type C clusters) alloy bimetallic clusters are prepared. Estimated relative reactivity per surface Pd atoms for the different clusters (c).
Temperature programmed reaction (TPR) has been performed over four different set-ups of metallic clusters (Fig. 3): On clean gold there is clearly no reactivity at all. On clean palladium clusters (directly deposited DD), 5nm size on average, ethylene (single peak at 220K, Fig. 3a) and benzene (270K peak, Fig. 3b) are produced. Subsequent deposition of palladium and gold clusters (resulting in clusters type A) leads to similar reactivity of the clean palladium. The most significant reactivity was found in the case of type C, alloy clusters. In this case higher reactivity towards ethylene formation and three different reactivity peaks for benzene formation are observed (270K, 460K and 650K), similar to reactivity on extended palladium surfaces. Overall reactivity was at least three times higher than that of all other Pd containing clusters.
3. Thermal stability of BLAG clusters
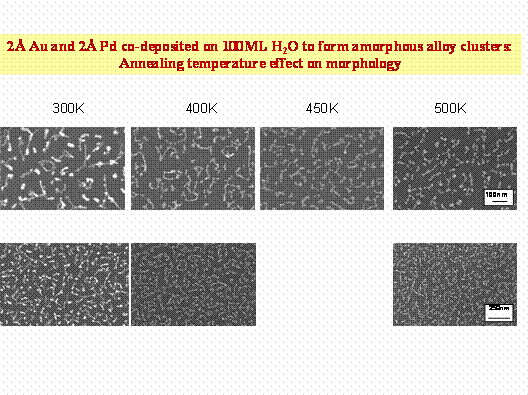
The thermal stability of gold, palladium and their bimetallic alloys was tested while residing on silica substrate. TEM images were utilized to monitor the clusters physical stability. Reactivity tests have monitored benzene formation following pre-annealing cycles. The effect of pre-annealing temperature on clusters morphology is displayed in Fig. 4.
Figure 4: Effect of annealing temperature on the morphology of Pd-Au amorphous alloy clusters prepared via BLAG, as indicated by TEM images.
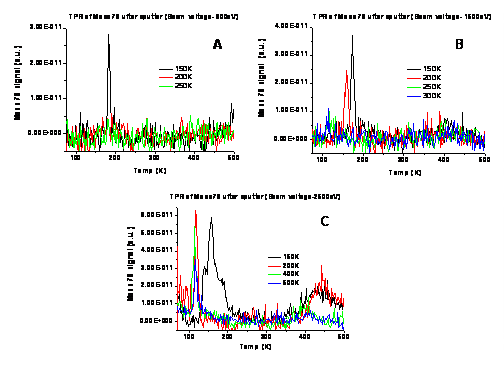
Figure 5: Reactivity tests of alloy Pd-Au clusters formed via type C BLAG method (benzene formation). The indicated temperatures are annealing values performed for each of the metallic clusters-silica samples. Sputtering of the silica substrate at 800 eV (A), 1500 eV (B) and 2500 eV (C) for 10 seconds preceded the BLAG deposition step.
The data in Fig. 5 reveals how relatively low annealing temperature already destroys the activity of these model Pd-Au catalysts after a single TPR cycle.
Annealing at temperature above 150K diminishes the reactivity on clean, non-modified SiO2/Si(100). The same happens in the case of sputter for 2 minutes at 800 eV Ar ion kinetic energy. Sputter at 1500 eV and 2500 eV cause enough surface damage and imperfections on the silica substrate to enhance adhesion of the subsequently deposited BLAG metallic clusters. Subsequent reactivity could be observed following anneal to 200K and 600K after sputter to 1500 eV and 2500 eV, respectively.
An interesting issue regarding sintering has been the substrate effect. Growing a thin film, about 5nm thick, of TiO2 via water as buffer layer, we could show significant reduction in density, due to sintering, of Ag clusters grown on the smooth TiO2 film compared with the clean SiO2 substrate.
Future studies utilizing XPS and conducting AFM will be performed to further characterize the atomic level structure of the defects. Damage by pure electrons will also be studied.