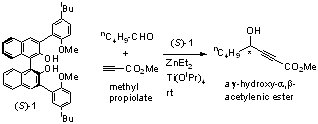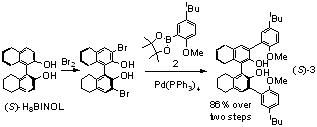Reports: AC1
45978-AC1 Development of Organocatalysts for the Asymmetric Alkyne Addition to Aldehydes and Applications in Organic Synthesis
Previously, we have found that a system composed of 1,1'-bi-2-naphthol (BINOL)-Ti(OiPr)4-ZnEt2-HMPA can catalyze the reaction of methyl propiolate with aromatic aldehydes at room temperature with very high enantioselctivity. However, this system shows lower enantioselectivity for the reaction of propiolate with aliphatic aldehydes. In order to improve the enantioselectivity of the BINOL-based catalyst for the asymmetric propiolate addition to aliphatic aldehydes, we have conducted a strucural modification of the BINOL ligand.
The 3,3'-bisanisyl substituted BINOL ligand (S)-1 is prepared and tested for the reaction of methyl propioate with pentyl aldehyde (Scheme 1). In the presence of ZnEt2 and Ti(OiPr)4 at room temperature, (S)-1 catalyzes this reaction to generate a g-hydroxy-a,b-acetylenic ester product with 62% yield and 85% ee. No Lewis base additive is needed for this reaction.
Scheme 1. Asymmetric addition of methyl propiolate to pentyl aldehyde catalyzed by (S)-1 in combination with ZnEt2 and Ti(OiPr)4.
In oder to futher improve the enantioselctivity of (S)-1, a partially hydrogenated BINOL ligand (S)-3 is synthesized (Scheme 2). This ligand contains a H8BINOL unit that has increased steric bulkiness due to the sp3 hybridized carbons on the partially hydrogenated naphthalene rings and has shown enhanced enantioselectivity in a number of catalytic processes. Compound (S)-3 can be readily prepared from (S)-H8BINOL in two steps in 86% yield. This ligand is examined for the reaction of methyl propiolate with aliphatic aldehydes.
Scheme 2. Synthesis of the H8BINOL-based ligand (S)-3.
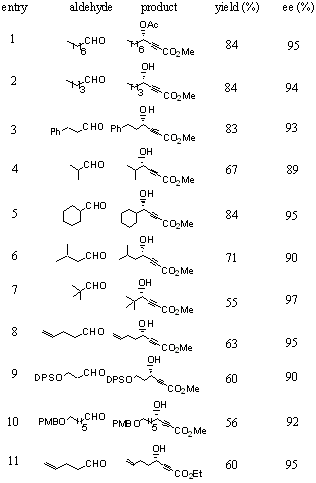
The conditions for the reaction of methyl propiolate with octyl aldehyde catalyzed by (S)-3 is explored (Table 1). As shown in the table, a large solvent effect is observed for the enantioselectivity of this reaction (entries 1-5). The more coordinative THF is found to be better than CH2Cl2, Et2O and toluene. The enantioselectivity in dioxane (87% ee) is found to be close to that in THF but in a lower yield (entry 4). It is also found that 50 mol % of Ti(OiPr)4 is optimal (entry 5). When no or only 10 mol % of Ti(OiPr)4 is used, no enantioselectivity is observed also with low yields (entries 7,8). Increasing the amount of Ti(OiPr)4 from 50 mol % to 100 mol % (entry 9) leads to reduced yield while maintaining the enantioselectivity. Decrasing or increasing the concentration of this reaction (entries 10,11) does not change the enantioselectivity, but diminishes the yield. Addition of Ti(OiPr)4 in the first step decreases the yield (entry 12). When the amount of the chiral ligand (S)-3 is increased from 10 mol % to 20 mol %, both the yield and ee are increased (84% yield and 95% ee, entry 13). Thus, entry 13 is identified as having the optimized conditions.
Table 1. Optimization of Conditions for the Reaction of Methyl Propiolate with Octyl Aldehdye Catalzyed by (S)-3.
|
entry
|
(S)-3
(mol %)
|
solvent (aldehyde concn M)
|
Ti(OiPr)4 (mol %)
|
yield (%)
|
ee (%) |
|
1
|
10
|
CH2Cl2 (0.1)
|
50
|
51
|
78
|
|
2
|
10
|
Et2O (0.1)
|
50
|
73
|
37
|
|
3
|
10
|
Toluene (0.1)
|
50
|
44
|
69
|
|
4
|
10
|
1,4-dioxane (0.1)
|
50
|
49
|
87
|
|
5
|
10
|
THF (0.1)
|
50
|
70
|
91
|
|
6
|
10
|
THF (0.1)
|
0
|
28
|
0
|
|
7
|
10
|
THF (0.1)
|
10
|
27
|
0
|
|
8
|
10
|
THF (0.1)
|
25
|
51
|
76
|
|
9
|
10
|
THF (0.1)
|
100
|
47
|
91
|
|
10 |
10
|
THF (0.2 )
|
50
|
65
|
90
|
|
11 |
10
|
THF (0.05)
|
50
|
40
|
90
|
|
12 |
10
|
THF (0.1)
|
50
|
48
|
90
|
|
13 |
20
|
THF (0.1)
|
50
|
84
|
95
|
The conditions of enter 13 in Table 1 are applied to the asymmetric reaction of methyl propiolate with various aliphatic aldehydes. As the results summarized in Table 2 show, good yields and excellent enantioselectivities are obtained for the reactions of linear, a-branched, and b-branched aliphatic aldehydes (entries 1-6). The more bulky trimethylacetaldehyde requires a higher loading of the ligand (40 mol %) and other reagents (entry 7). Functionalized aliphatic aldehydes also give high enantioselectivity (entries 8-10). Ethyl propiolate reacts with 4-pentenal (entry 11) with the same high enantioselectivity as methyl propiolate (entry 8). The product in entry 11 is determined to have S configuration. The absolute configuration of the g-hydroxy-a,b-acetylenic esters obtained by using (S)-3 as the catalyst is opposite to that by using (S)-BINOL. This demonstrates that this structurally modified BINOL ligand provides very different stereocontrol from BINOL.
Table 2. Addition of Alkyl Propiolates to Aliphatic Aldehydes Catalyzed by (S)-3.
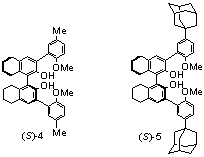
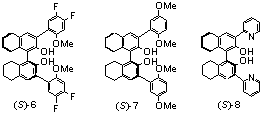
We have studied the steric effect of the t-butyl groups of (S)-3 by replacing them with the Me and adamantanyl groups in (S)-4 and (S)-5. These ligands (10 mol%) are used to catalyze the reaction of methyl propiolate with octyl aldehyde under the conditions of entry 5 in Table 1, and they give the desired products with 90% ee in both cases and in 59% and 63% yields respectively. The enantioselectivity of (S)-4 and (S)-5 is very close to that of (S)-3 and the size of the alkyl substituents has little influence on the enantioselectivity.
We have also studied the electronic effects by using ligands (S)-6 and (S)-7. Under the conditions of entry 5 in Table 1, (S)-6 containing the electron-withdrawing fluorine groups shows reduced enantioselectivity (70% ee) for the reaction of methyl propiolate with octyl aldehyde. However, (S)-7 containing the electrondonating methoxy groups gives a better ee of 86%, closer to that of (S)-3. Ligand (S)-6 also gives lower yield (34% than (S)-7 does (52%). This indicates that the Lewis basic MeO groups of these ligands should be involved in the coordination with the Lewis acidic metal centers and contribute to the catalytic process. Ligand (S)-8 where pyridine is used to replace the 3,3'-bisanisyl groups of (S)-3 gives both poor enantioselectivity (34% ee) and poor yield (48%) for the reaction of methyl proopiolate with octyl aldehyde. This also shows the importance of the MeO groups of (S)-3.