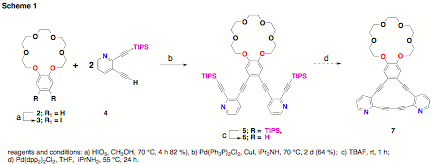Reports: B4
46543-B4 Ion Sensitive Push-Pull Pyridoannulenes
 The goal of this work is for
undergraduates to synthesize a series of annulenes and test their spectroscopic
properties in the presence of various metal cations. This report will update
the status of the project since the last report. With our successful
synthesis of annulene 1 during the first summer, we sought to prepare isomeric annulene 7. Unfortunately, this synthesis was
not successful. The proposed synthesis is shown in Scheme 1.
The goal of this work is for
undergraduates to synthesize a series of annulenes and test their spectroscopic
properties in the presence of various metal cations. This report will update
the status of the project since the last report. With our successful
synthesis of annulene 1 during the first summer, we sought to prepare isomeric annulene 7. Unfortunately, this synthesis was
not successful. The proposed synthesis is shown in Scheme 1.
First, commercially available benzo-18-crown-6 was treated with periodic acid in methanol. The result of this reaction was the facile isolation of diiodide 2 in high yield and in sufficient purity that no contaminants could be detected by either 1H or 13C NMR spectroscopy. Compound 4 was prepared from commercially available 2-bromo-3-pyridinol in three steps using a procedure previously published from our lab. Benzo-crown 2 underwent Sonogashira coupling with two equivalents of 4 to yield 5 in 64 % that also contained unidentified impurities. Several attempts were made at this reaction. Each time this reaction was performed these impurities were noted. These impurities were slightly less polar by thin layer chromatorgraphy and clearly visible in both the aromatic and alkoxy regions of the 1H NMR spectra. Assuming that the impurities were the result of incomplete Sonogashira coupling, attempts were made to push the reaction to completing by subjecting the crude material to additional 4. However, 1H NMR spectra indicated the same ration of impurities relative to products, even after the reaction. Thus, a plethora of techniques were used to try to separate the impurities from the desired product. This included normal-phase flash chromatography in a variety of solvents, recrystallization, tituration, normal and reverse-phase HPLC and reverse-phase column chromatography. We are now forced to re-examine the synthetic route either closing the crown ether as one of the final steps or a more linear route approach where (3-cyanopropyl)-dimethylsilyl acetylene is attached to the crown ether and pyridine moieties attached in a separate step.
This research has had a significant impact on all who participated in the project, as well as the PI, and other students who were not specifically involved in this area. Through presentations and the experiments themselves, we all learned a great deal with respect to handling these crown compounds. The summer had a significant impact on the PI with respect to the desired to continue working and expanding the research in this area. All of the senior level students involved in the project benefited from the experience and were motivated to seek post graduate training, one now having been accepted to graduate school while two others have applied to pharmacy school and one to medical school. The one student, who is a rising junior, has decided that he is going to change his major to chemistry and wishes to attend chemistry graduate school.