Reports: G7
46864-G7 Templating Ion-Conducting Network Membranes Using Tapered Block Copolymers
The need for clean and sustainable energy sources is growing rapidly, and electrochemical devices such as batteries and fuel cells are considered promising solutions. Polymer electrolyte membranes for use in lithium batteries and fuel cells require high ionic conductivities to decrease the internal potential losses. Besides ionic conductivities, adequate mechanical strength is necessary to prevent short-circuiting between electrodes and to reduce dendrite formation in lithium batteries.
To achieve the above requirements for lithium ion batteries, block copolymers containing conducting blocks like poly(ethylene oxide), and complementary blocks, have been employed to vary the morphology and properties of conducting materials. The self-assembly of these copolymers permits the design of flexible and sturdy membranes that contain conducting channels for ion transport.
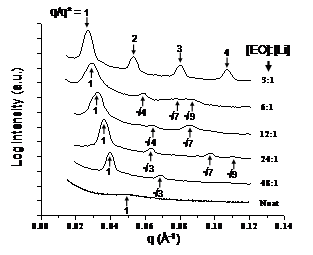
For our studies to date, we have employed cylinder-forming poly(styrene-b-ethylene oxide) [PS-PEO] block copolymers, where the polymer electrolyte cylinders were formed via a self-assembly process inside a polystyrene matrix.1,2 The three lithium salts used were, LiClO4, LiCF3SO3, and LiAsF6. The block copolymers were doped with salts at ether oxygen to lithium cation ([EO]:[Li]) ratios ranging from 48:1 to 3:1. Using transmission electron microscopy (TEM), differential scanning calorimetry (DSC), and temperature-controlled small-angle X-ray scattering (SAXS), we evaluated the effects of lithium salt counterions on the phase behavior of PS-PEO diblock copolymers. Salt-doping leads to an increase in segregation strength (as specifically quantified by ceffN [where ceff is the effective Flory interaction parameter and N is the effective degree of polymerization]) for all PS-PEO:LiX (X=ClO4-, CF3SO3-, AsF6-) systems as illustrated by increases in domain spacings (d*), where q* = 2p/d*.1 Figure 1 shows in-situ SAXS profiles of PS-PEO:LiClO4 as a function of salt-doping ratio ([EO]:[Li]) obtained at 166 oC. As the lithium salt concentration increases from 48:1 to 3:1, the primary scattering peak shifts to smaller q values (larger domain spacings). This shift is expected as salt-doping has been previously shown to induce chain stretching. The PS-PEO:LiClO4 samples with doping ratios from 48:1 to 6:1 exhibit a hexagonally-packed structure with Bragg reflection peaks located at q*, √3q*, √4q*, √7q*, and √9q*, while the 3:1 material exhibits a lamellar structure with Bragg reflection peaks located at q*, 2q*, 3q*, and 4q*. Thus, LiClO4-doping in PS-PEO causes a cylinder to lamellae phase transition between the 6:1 and 3:1 LiClO4-doping ratios. The existence of the hexagonally packed cylinder and lamellar nanostructures was corroborated by TEM.1
We also examine the thermal behavior of these three PS-PEO/salt systems. Through DSC analysis, we find that the phase behavior of the comparable salt-doped PEO domains is consistent with binary phase diagrams for the salt-doped PEO homopolymers. This implies that all of the lithium salt resides in the PEO domains at doping ratios up to 3:1. Furthermore, crystallization of the PEO salt-complexes above the glass transition temperature of the poly(styrene) block leads to discontinuous changes in domain spacing as a function of temperature.2
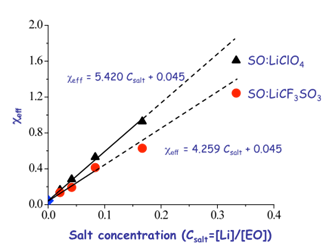
Finally, we find a linear relationship between ceff and salt concentration in each of our salt-doped PS-PEO systems, where ceff is estimated from the strong segregation theory. By comparing the three lithium salts, we note that LiAsF6-doping shows the largest increase in segregation strength at a given salt concentration, which we relate to the weaker Lewis base character of the AsF6- relative to the other two lithium counterions. This difference in counterion activity leads to differences in the domain spacing (and segregation strength) between the various copolymer:salt complexes, and ultimately differences in their phase behavior at similar lithium ion concentrations.1
We have recently constructed a conductivity apparatus that allows us to perform simultaneous SAXS and conductivity measurements on our doped samples at various temperatures. Studies to examine the effect of morphology and domain size on conductivity are currently underway.
Additionally, we have begun the synthesis and exploration of tapered block copolymers. In our initial work, we examine tapered block copolymers consisting of symmetric poly(isoprene-b-styrene) block copolymers. We recently reported a synthetic strategy that allows us to manipulate the interfacial region between blocks and control ordering transitions in poly(isoprene-b-styrene) [P(I-S)] block copolymers.3 A library of copolymers was prepared with various interfacial modifications (tapers) to examine the effect of interfacial composition on copolymer self-assembly. The morphological characteristics of the self-assembled structures are investigated using SAXS, TEM, and dynamic mechanical analysis (DMA). Normal and inverse tapered block copolymers, containing approximately 15-35 vol % tapered material, show a measurable decrease in the order-disorder transition temperature (TODT) relative to the corresponding non-tapered diblock copolymers; with the inverse tapered materials showing the greatest deviation in TODT.3 Additionally, TODT was inversely related to the volume fraction of the tapered region in both normal and inverse tapered copolymer materials.3
Figure 1. SAXS profiles of LiClO4-doped PS-b-PEO as a function of salt concentration. Samples were held under vacuum and at 166 oC for 1 h prior to data acquisition. The diffraction peaks are identified by q/q*, where q* is the primary peak. Curves are shifted vertically for clarity.
Figure 2. ceff versus salt concentration ([Li]/[EO]) at 166 oC. The ceff values for salt-doped samples are calculated using d* data obtained from SAXS experiments and the relationship between d* and c in strong segregation regime. The solid lines were fit using the cylinder-forming samples in each system, and the lines were force fit through c of the neat sample. The fits, based on the cylinder-forming data, are extended to the lamellar samples (dash lines).
1. Young, W.; Epps, T. H., III, Macromolecules, 2009, 42(7); 2672-2678 Phase Behavior and Interaction Parameters in Salt-Doped Polymer Electrolytes: Counterion Effects.
2. Young, W.; Brigandi, P. J.; Epps, T. H., III, Macromolecules, 2008, 41(17); 6276-6279 Crystallization-Induced Lamellar-to-Lamellar Thermal Phase Transitions in Salt-Doped Polymer Electrolytes.
3. Singh, N.; Tureau, M. S.; Epps, T. H., III, Soft Matter, 2009, 5(23) 4757-4762. Manipulating Ordering Transitions in Interfacially Modified Block Copolymers.
4. Singh, N.; Tureau, M. S.; Epps, T. H., III, 2009 (submitted, Journal of the American Chemical Society) Interfacially Modified Triblock Copolymers using a Combination of Anionic and Atom Transfer Radical Polymerization (ATRP).