Reports: GB4
46022-GB4 Influence of Ring Size and Substituents on the Cyclopropylcarbinyl Radical Fragmenttion
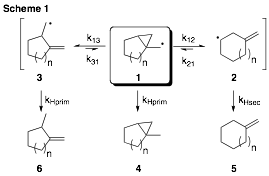
During the timeframe of this grant, we have explored the thermokinetics of fragmentation of 1-bicyclo[n.1.0]alkylmethyl radicals (e.g., 1) and related systems. Our investigations encompassed several homologues of the prototype system 1 (Scheme 1), with additional studies aimed at benzofused derivatives of this framework. We initially investigated the five-membered ring (1, n=1) which promptly resulted in a publication. Due to the prominence of this particular system in many early cyclopropylcarbinyl radical explorations, it seemed appropriate to disseminate the five-membered ring thermokinetic study as a standalone report. This paper includes five undergraduate research student co-authors.
Our studies of higher homologues of this system have also been fruitful (Table 1) and a manuscript is presently in preparation. The rate expressions for the seven-membered ring (n=3) were determined to be log(k12/(s-1)) = 13.57 – 8.96/q and log(k13/(s-1)) = 13.23 – 7.55/q; for the eight-membered ring (n=4), log(k12/(s-1)) = 12.22 – 6.49/q and log(k13/(s-1)) = 12.54 – 6.70/q; for the ten-membered ring (n=6), log(k12/(s-1)) = 11.55 – 3.72/q and log(k13/(s-1)) = 13.34 – 7.99/q. These analyses were performed over a temperature range of –78 to 56 ûC using thiophenol as a trapping agent and employing the competition method. Additionally, through the use of a slower radical trapping agent (tri-n-butylstannane), we have also established the thermodynamically favored product in each of these cases.
Table 1. Fragmentation rates 298 K.
|
log k13
|
n
|
ring size
|
log k12
|
|
6.84
|
1
|
5
|
8.92
|
|
7.70
|
2
|
6
|
8.15
|
|
7.70
|
3
|
7
|
7.01
|
|
7.63
|
4
|
8
|
7.46
|
|
7.48
|
6
|
10
|
8.82
|
|
in progress
|
8
|
12
|
in progress
|
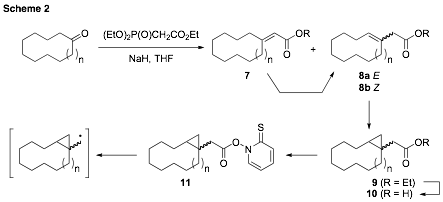
We had initially planned to disseminate the results for the two medium-ring substrates (n = 3 and 4) together, and then report larger ring sizes (n = 6, 8, 11) in a separate submission. However, the larger rings proved to be more challenging targets for a number of reasons. First, the twelve- and fifteen-membered rings presented solubility issues for some of the steps along the synthetic sequence. Also, as anticipated for the larger ring sizes (n > 4), a mixture of E and Z isomers was observed en route to the radical precursors (Scheme 2). This would ultimately lead to stereoisomers of the cyclopropylcarbinyl radicals (1) entering into the reaction manifold. It should be noted, however, that the ring-opened hydrocarbon products clear this stereochemical feature, irrespective of their origins (E or Z), permitting a simple GC-MS analysis comparable to the smaller ring sizes. The resulting kinetic analysis would then be a composite of the rates of the individual E and Z isomers. Additionally, for the larger rings, isomerization of the a,b-unsaturated ester to the b,g-unsaturated ester (e.g., 7 ¨ 8) could not be effected to any satisfying degree through a variety of methods.
Despite these challenges, we have recently achieved preparation of the ten-membered ring PTOC ester (11, n=1). This has been accomplished by modifications to the Wadsworth-Emmons reaction that resulted in securing exclusively one of isomeric b,g-unsaturated esters. It is presently unknown whether it is the E or Z isomer, but it is expected that this will be readily discerned by NMR. The remainder of the sequence was uneventful and adequate amounts of the radical precursor were prepared in order to carry out the thermokinetic analysis. We are presently completing characterization of the compounds associated with this sequence.
The success of the cyclodecyl system has given us cause to re-evaluate our approach to the twelve-membered ring, and we are currently making substantial progress along this avenue. On the other hand, we have decided to abandon the fifteen-membered ring due to the aforementioned difficulties. At this stage, we have decided to abandon the original concept of splitting up the data and are now planning on combining all of the medium- and large-ring data into one manuscript. If we achieve success with synthesis of the twelve-membered ring entry, then it will also be included.
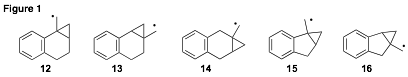
Our investigations have also included the exploration of benzofused derivatives (Figure 1). We encountered an unfortunate fate with radical 12, in that two of the three hydrocarbon products exhibited identical GC retention times among several different capillary columns. Synthetic efforts toward investigating radical 14 have proven themselves to be more challenging than originally anticipated and we have relegated this target to a lower priority. Studies toward radical 15 are still underway. Alternatively, radicals 13 and 16 have yielded some highly interesting results.
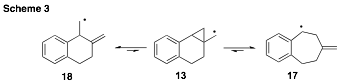
As expected, we have found that radical 13 fragments exceptionally fast along the ring-expansion pathway to give intermediate 17 (4.2 x 109 s-1 at 298K, Scheme 3). The rate expression for 13 ¨ 17 was determined to be log(k12/(s-1)) = 11.02 – 1.92/q; for 13 ¨ 18, log(k13/(s-1)) = 10.45 – 2.88/q; Undoubtedly, the benzylic stabilization enjoyed by radical 17 contributes significantly to this rate-enhancement. It was necessary to use the very fast trapping agent PhSeH (kT = 109 M-1s-1) to establish these fragmentation rates. When tri-n-butylstannane is used as the reducing agent, the ring-expansion product resulting from reduction of 18 is produced exclusively.
Of greater interest is cyclopropylcarbinyl radical 16. When this radical is generated in the presence of PhSeH at low temperature (-78 ûC) the ring-expanded product is formed exclusively, with no evidence of any unopened cyclopropane! We currently estimate this fragmentation along the shared bond of the system to be occurring at a rate ³ 1011 s-1. This result is not completely unexpected considering the unusually fast fragmentation rate of ring-expansion of the prototype five-membered ring (1, n = 1, k12 = 8.4 x 108 s-1 at 298 K) which is embedded in this system. This system will be welcomed among some of the fastest cyclopropylcarbinyl rearragements known in the literature. The ring-expansion pathway is also the exclusive outcome when equilibrating conditions with the slower tri-n-butylstannane trapping agent are employed. A manuscript reporting the results of the benzofused derivatives is planned for submission.