Reports: B1
48367-B1 Beta-Hydroxysalicylhydrazones: Chiral, Non-racemic Tridentate Catalysts for Asymmetric Synthesis
In this granting period, my research group has been focused on the synthesis and development of chiral, non-racemic beta-hydroxysalicylhydrazone. Hydrazones have recently been reported as useful catalytic ligands in the addition of diethylzinc to aldehydes. The process is driven via coordination of diethylzinc with an appropriate ligand, most likely derived from a chiral, non-racemic beta-amino alcohol. Among the variety of ligands described in the chemical literature which were applied in the asymmetric addition of diethylzinc to variety of aldehydes, salicylhydrazone represents a category of compounds that have not been studied extensively.
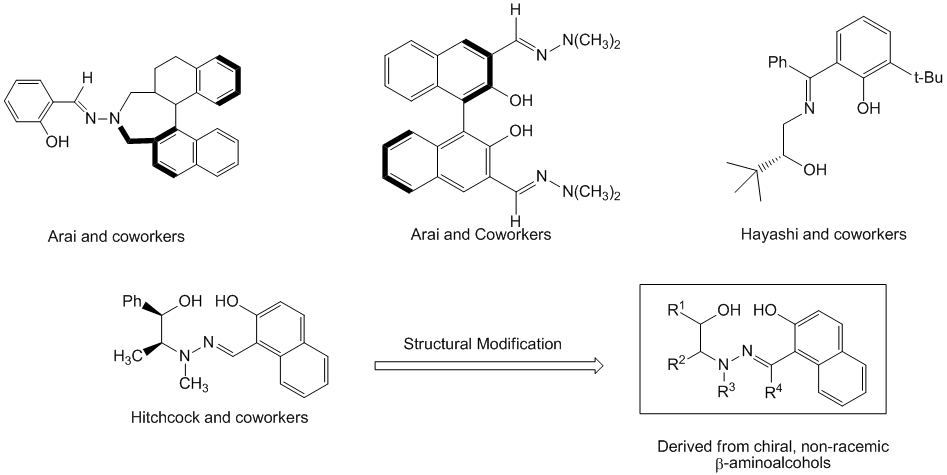
The most relevant literature on salicylhydrazone family of ligands has been developed by Arai and co-workers. They developed two salicylhydrazone derivatives using a binaphthyl template. Of the two frameworks that were developed, the first contained only one salicylhydrazone moiety and the second contained two salicylhydrazone moieties (Figure 1). When employed in the asymmetric 1,2-addition of diethylzinc, these ligands afforded enantiomeric ratios as high as 90.0:10.0. In similar work, Hayashi and coworkers disclosed that a beta-hydroxysalicylhydrazone derived from tert-leucinol proved to be effective catalyst for the asymmetric addition of diethylzinc to aldehydes.
Previously in my research group, a series of tridentate β-hydroxysalicylhydrzone ligands were prepared from (1R,2S)-ephedrine and (1R,2S)-norephedrine and then tested in catalytic asymmetric addition of diethylzinc to aldehydes (Figure 1). These reactions exhibited very good enantioselectivity with enantiomeric ratios ranging from 89:11 to 96:4. In following the trajectory of this work, we developed an interest in the synthesis and application of other derivatives of the beta-hydroxysalicylhydrazone families of compounds having significant structural differences that could be exploited for enhanced enantioselectivities (Figure 1).
Figure 1. beta-Salicylhydrazone development.
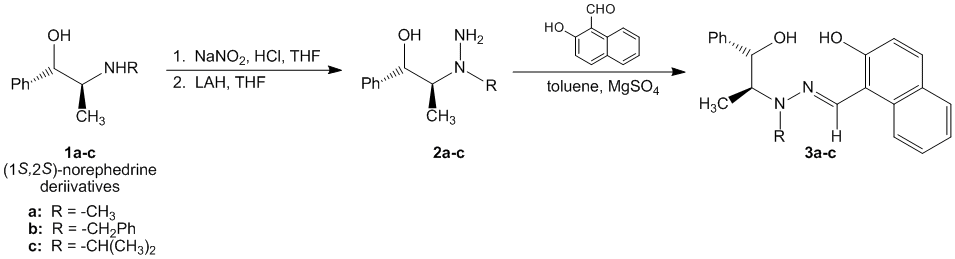
We launched this work by the synthesis of a series of b-hydroxsalicylhydrazones derived from pseudoephedrine and pseudonorephedrine. This was accomplished by the formation of the corresponding N-nitrosamines, reduction to the hydrazine form and subsequent condensation with 2-hydroxy-1-naphthaldehyde (Scheme 1).
Scheme 1. Ephedra based beta-hydroxysalicylhydrazones.
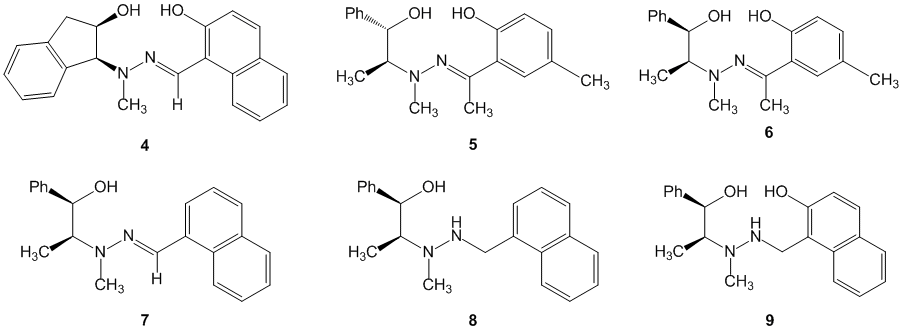
In addition to these ligands, we also synthesized a series of related ligands that possessed the salicylhydrazone core (Figure 2). Ligand 4 was prepared to study the catalysis process when the ligand has restricted rotation about the b-amino alcohol component. Ligands 5 and 6 were prepared for the purpose of determining the viability of using ketones rather than aldehydes as condensation partners. Ligands 7 and 8 were prepared for the purpose of exploring the successful use of these ligands as catalyst absent the conformationally restricting imine bond.
Figure 2. Synthetic variations of the beta-hydroxysalicylhydrazone motif.
The asymmetric 1,2-addition process of diethylzinc to 2-naphthaldehyde was conducted as a test of the efficacy of these ligands (Table 1). The enantioselectivities of these reactions ranged from 13 to 68%ee. The results of the testing were not optimal but did point my research in another more successful direction, that of designing simpler ligands that would allow for synthetic studies and improved enantiomeric excesses.
Table 1. Catalytic asymmetric addition of diethylzinc to 2-naphthaldehyde.
|
entry
|
catalyst
|
yield (%) a
|
enantiomeric ratio
S:R, (%ee)b
|
absolute
config.c
|
|
1
|
3a
|
90
|
63.0:37.0 (26) |
S
|
|
2
|
3b
|
46
|
69.0:31.1 (38)
|
S
|
|
3
|
3c
|
47
|
63.4:37.6 (26) |
S
|
|
4
|
4
|
90
|
63.4:36.6 (27) |
S
|
|
5
|
5
|
58d
|
65.6:34.4 (31)
|
S
|
|
6
|
6
|
66 d
|
43.6:56.4 ( 13)
|
R
|
|
7
|
7
|
38
|
33.3:66.7 (33)
|
R
|
|
8
|
8
|
96
|
16.0:84.0 (68)
|
R
|
|
9
|
9
|
92
|
42.7: 57.3 (15)
|
R
|
| aAll reaction went to completion as determined by 1H NMR spectroscopy and CSP HPLC. bThe enantiomeric ratio (er) values were determined via CSP HPLC using a Chiralcel-OD column. cThe configuration was determined by comparison of the literature values. dCombined yield of addition and reduction product.
|
||||
N-Pyridylmethyl substituted Ephedra derivatives were synthesized by either direct alkylation or reductive alkylation of (1R,2S)-norephedrine, (1S,2S)-pseudonorephedrine, and (1R,2S)-ephedrine (Scheme 2). These derivatives were then employed in the asymmetric addition reaction with diethylzinc and aldehydes and diphenylphopshinoylimines. The use of the Ephedra family allowed for a systematic evaluation of the contribution of the pyridyl group.
Scheme 2. a-N-Pyridylmethyl substituted Ephedra based ligands.
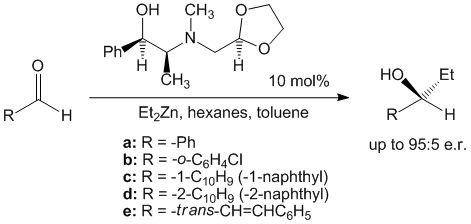
In another body of work, an investigation of the impact of oxygenated side chains in Ephedra compounds on the catalytic asymmetric addition of diethylzinc to aldehydes were conducted (Scheme 3). (1R,2S)-Ephedrine and (1S,2S)-pseudoephedrine were alkylated with either alkyl halides or beta-alkoxyalkyl halides to afford a series of ligands. These compounds were employed in the enantioselective addition of diethylzinc to a variety of aldehydes. It was determined that the presence of oxygen could have a negative effect in terms of obtaining high levels of enantiomeric discrimination, but the effect is diminished with higher levels of substitution near the oxygen.
Scheme 3. Oxygenated side-chains in Ephedra compounds.
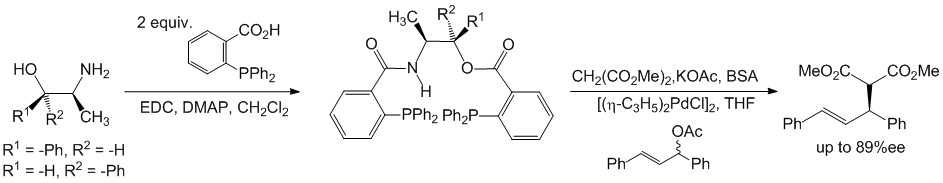
The diethylzinc addition reactions were of interest for our group, provided technical training for graduate students, training and support for undergraduate students, and an ACS project SEED student. However, there was an interest in pursuing a different line of synthetic chemistry as the diorganozinc chemistry was very specific in its application and could not be widely applied to different synthetic problems. It was during this time that A. J. Burke and coworkers had published an article in Tetrahedron: Asymmetry that inspired our group to explore the use of Ephedra derivatives as templates for making phosphine ligands. Thus, (1R,2S)-norephedrine and (1S,2S)-pseudonorephedrine were reacted with two equivalents of o-(diphenylphosphino)benzoic acid, two equivalents of 1-ethyl-3-dimethylaminopropyl carbodiimide, and a catalytic amount of DMAP to afford a series of phosphine ligands (Scheme 4). We learned that these easily prepared Ephedra derivatives could indeed serve as effective ligands in the palladium catalyzed asymmetric allylic alkylation of dimethyl malonate and afforded product enantioselectivities up to 89% ee. Our current efforts are now directed towards using the Ephedra alkaloids and other chiral, non-racemic beta-amino alcohols.
Scheme 4. Ephedra based o-(diphenylphosphino)benzoyloxy o-(diphenylphosphino)benzamide.