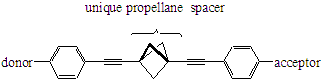Reports: GB4
46532-GB4 Synthesis and Characterization of Novel Donor/Acceptor Molecules Based on Propellanes: A Probe of Nonclassical Conjugation
A new class of aromatic donor/acceptor molecules based on 1,3-diethynylbicyclo[1.1.1]pentane (diethynylpropellane) spacers has been prepared. The molecules provide an interesting probe of nonclassical conjugation through the bicyclo[1.1.1]pentane (propellane) unit. We have prepared six different targets, and UV-vis and fluorescence spectral data indicate significant interaction between donor and acceptor groups.
This study provides the first experimental evidence for charge transfer between donor (D) and acceptor (A) groups linked by diethynylpropellanes and effectively demonstrates nonclassical conjugation through the bicyclo[1.1.1]pentane (propellane) unit. Charge transfer is believed to be due to electronic interactions through the strained propellane arising from the increased p-character of the bent C-C bonds: this interaction is largely due to through-bond interactions (pi-sigma-pi conjugation). The molecules exhibit substantial charge transfer and may find potential applications in molecular electronics, optics, and energy transfer. This report describes our progress on the funded work.
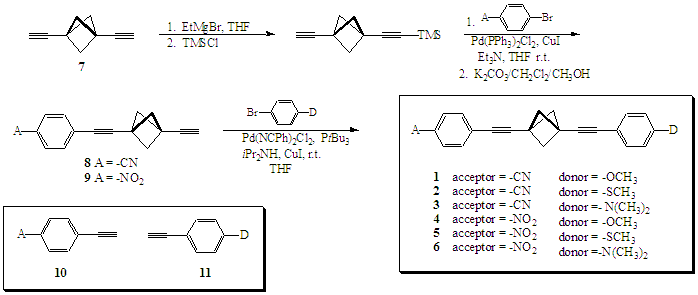
Synthesis of the Targets: Synthesis of the targets has proved most challenging. We have prepared six of the target D/A molecules using sequential Sonogashira coupling1 of the appropriate donor or acceptor substituted halobenzenes to 1,3-diethynylbicylco[1.1.1]pentane 7. (Scheme 2) Our route is based on Michl's earlier syntheses of related symmetrical derivatives.2 The required 1,3-diethynylbicyclo[1.1.1]pentane2,3 was prepared in four steps from commercially available 1,1-dibromo-2,2 -bis(chloromethyl)cyclopropane or in eight steps from pentaerythritol.4 In our hands, mono-TMS protection of the 1,3-diethynylbicyclo[1.1.1]pentane prior to palladium coupling proved to be the most efficient route. Protection of 7 was accomplished using 1 eq. of EtMgBr in THF followed by addition of chlorotrimethylsilane. Silylation always gave a small amount of bis-silylated product which could be easily removed by chromatography after the first Pd coupling reaction.
Scheme 1
In addition to the targets, a full range of unlinked p-substituted phenylacetylenes (10 and 11) were prepared (Scheme 1). These molecules were required in order to compare the isolated donor and acceptor chromophores to the target molecules.
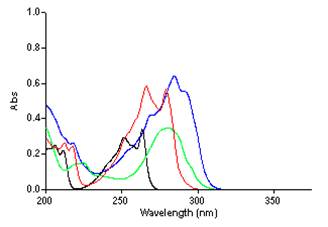
UV-vis and Fluorescence Studies: UV-vis and fluorescence spectroscopy were used to characterize the electronic and optical properties of the propellane based targets and provide evidence of significant interaction through the propellane spacer. A comparison of the UV-vis spectra of the targets with the corresponding p-ethynylbenzenes and the appropriate monoaryl substituted derivatives 8 and 9 demonstrates the extent of communication between the donor/acceptor groups. Figure 1 contrasts absorption spectra for the CN/SMe derivative 2 and is representative. The propellane based targets and the monoaryl derivatives show longer wavelength absorptions of higher intensity (epsilon >104) when compared to p-ethynylbenzenes. This increase in the molar absorptivity is evidence of a highly polarized excited state. Furthermore, the absorption maxima are sensitive to the nature of the substituent groups. Stronger donors and acceptors move the absorption maxima to longer wavelength: CN/SMe (2) 284 nm < NO2/SMe (5) 289 nm < NO2/NMe2 (6) 298 nm.
Figure 1. UV-vis Spectra of 2 in Cyclohexane
Overall, the molecules' absorption maxima exhibit minimal solvent dependence. Surprisingly, there is a small blue shift (< 5nm) in acetonitrile relative to cyclohexane for the targets and monoaryl derivatives. A similar blue shift in polar solvent was seen for the related p-CN/p-SMe substituted diphenylethyne, diphenylbutadiyne, and 1,4-bis(p-phenylethynyl)benzene.5 Fine structure observed in cyclohexane is lost when spectra are recorded in the more polar acetonitrile.
Absorption maxima for propellane based donor/acceptor systems are much lower than those for the corresponding diphenylethynes, diphenylbutadiynes, and 1,4-bis(p-phenylethynyl)benzenes because the donor and acceptor groups are not directly conjugated. However, the intensity of the absorption is comparable (based on the extinction coefficients) and the shift to longer wavelength relative to the individual p-substituted ethynylbenzenes supports interaction through the propellane spacer.
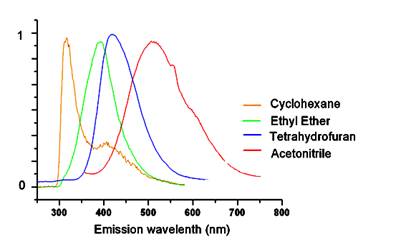
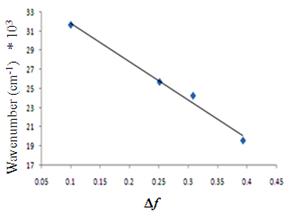
The fluorescence spectra show significant charge transfer between the donor and acceptor groups. For example, the emission spectra for 1 show a dramatic solvent effect (Figure 2). The emission maxima undergo bathochromic shift with increasing solvent polarity. Data for compound 1 were plotted using the Lippert equation6 and a good correlation was found between the emission intensity and solvent polarity parameter, Df (Figure 3). The large, negative slope suggests this target undergoes a substantial molecular dipole change between the ground and excited state. Clearly, interaction through the propellane spacer is demonstrated by this dramatic solvatochromic emission! We have seen similar behavior for the CN/SMe target 2 and are in the process of examining the other systems for solvatochromism.
Figure 2. Normalized emission spectra for 1 in solvents of different polarity. Excitation at 270 nm.
Figure 3. Lippert plot for 1 in solvents of varying polarity. R2= 0.988, slope= -39,000 cm-1
Impact on Undergraduate Education: Funding from the ACS-PRF has provided research stipends for six undergraduate students and the opportunity for two other students to contribute to this project through the purchase of the required chemicals and supplies to carry out the work. The project is challenging and has provided good training to students by exposing them to a wide variety of synthetic and analytical techniques. Students have been engaged in all aspects of the project which has been rewarding experience for all of ussome students are now planning to pursue graduate studies in chemistry!
Literature Cited:
(1) (a) Takahashi, S.; Kuroyama, Y.; Sonogashira, K.; Hagihara, N. Synthesis 1980, 627-630. (b) Hundertmark, T.; Littke, A.F.; Buchwald, S.L.; Fu, G.C. Org. Lett. 2000, 2, 1729-1731.
(2) Schwab, P.F.H.; Noll, B.C.; Michl, J. J. Org. Chem. 2002, 67, 5476-5485.
(3) Bunz, U.; Szeimies, G. Tetrahedron Lett. 1989, 30, 2087-2088.
(4) (a) Semmler, K.; Szeimies, G.; Belzner, J. J. Am. Chem. Soc. 1985, 107, 6410-6411. (b) Lynch, K. M.; Dailey, W. P. Org. Synth. 1997, 75, 98-105. (c) Kaszynski, P.; Michl, J. J. Org. Chem. 1988, 53, 4593-4594. (d) Levin, M. D.; Kaszynski, P.; Michl, J. Org. Synth. 2000, 77, 249-253. (e) Lynch, K. M.; Dailey, W. P. Org. Synth. 1997, 75, 89-97.
(5) (a) Khundkar, L. R.; Stiegman, A. E.; Perry, J.W. J. Phys. Chem. 1990, 94, 1224-1226. (b) Biswas, M.; Nguyen, P.; Marder, T. B.; Khundkar, L. R. J. Phys. Chem. A. 1997, 101, 1689-1695.
(6) Lippert, Von E. Z. Elektrochem. 1957, 61, 962.