Reports: G5
48672-G5 Synthetic Routes to Porous Polymers Containing Borazine and Diazaborole Building Blocks
For the past year we have been concerned with the preparation and characterization of borazine-linked polymers (BLPs) to prove that such polymers are synthetically viable and would have interesting gas storage and separation properties. The following synthetic routes were successfully evaluated and proved to be effective in BLP synthesis: (i) Thermolysis of arylamine-borontrihalide adducts and (ii) Homogeneous and heterogeneous dehydrogenation of amine-borane adducts. The overall results will be communicated in more details shortly.1,2
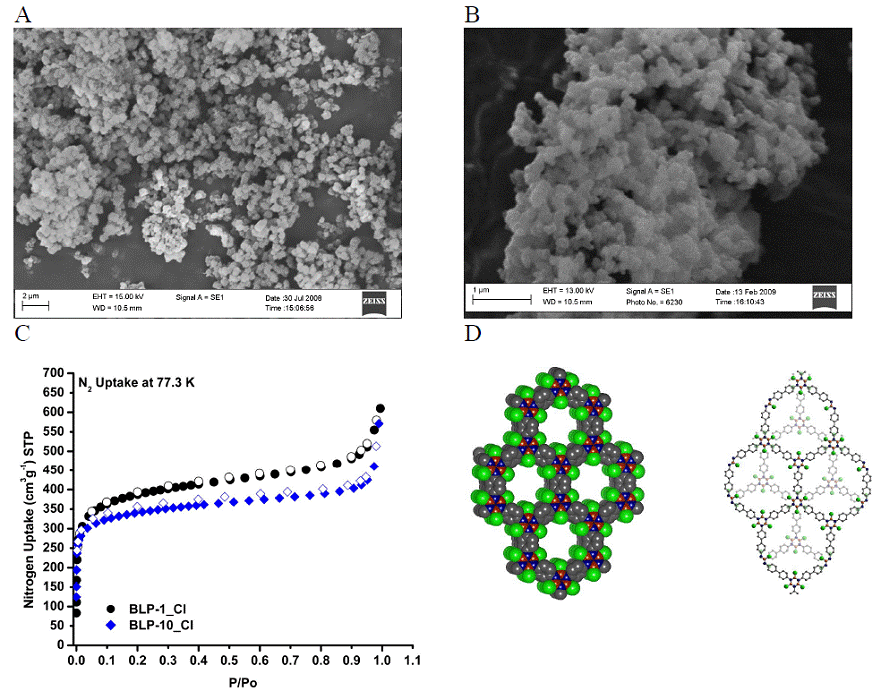
The preparation of BLP-1_Cl and BLP-10_Cl was carried out by thermolysis of BCl3-phenylenediamine and BCl3-benzidine adducts. The architectural stability, chemical composition, and phase purity were established using a combination of spectral and analytical methods as well as porosity measurements. The activation process involved washing with anhydrous CH2Cl2 followed by heating at 200 ºC at 10-5 torr for 24-48 hrs. The FT-IR spectra show considerable depletion of the stretching and bending bands for amine protons which usually appear around 3420 cm-1 and 1600 cm-1, respectively, and the appearance of several new bands at 1487, 1406, and 819 cm-1 which are characteristic of the B3N3 ring. TGA indicated that these polymers lose trapped solvents, dichloromethane and toluene, around 200 ºC and start to decompose at ~450 ºC under nitrogen. The initial weight loss (30 wt%) is indicative of their porous nature. Phase purities of all materials were examined using SEM and TEM imaging which showed that these polymers exist as irregular particles of about 0.5 µm.
So far, BLP-1_Cl and BLP-10_Cl are amorphous and their solid-state packing cannot be investigated by powder X-ray diffraction methods. To gain insight into the expected porosity of BLP-1_Cl and BLP-10_Cl, we considered two solid-state packings where the borazine rings adhere to the expected eclipsed conformation as in the case of boron nitride or a staggered conformation as in graphite. These models were constructed using Materials Studio Visualizer. Due to the nature of the trigonal-planar building blocks used to construct BLP-1_Cl and BLP-10_Cl, the expected solid-state packing will have two possibilities: graphite (gra, P63/mmc) where layers are slipped or boron nitride (bnn, P6/mmm) where layers are eclipsed as shown in Figure 1F. Either packing will result in porous networks. To verify the chemical connectivity of boron and its geometric environment we carried out solid-state 11B MQMAS experiments which show values that are consistent with chemical shifts reported for the three-coordinate boron centers of B-trichloroborazine. Additionally, the data for BLP-1_Cl revealed the presence of one signal, which is consistent with an eclipsed confirmation, while the data for BLP-10_Cl containing two signals may arise as a result of a staggered conformation placing the boron atoms in two different spatial environments.
Figure 1. SEM images of BLP-1_Cl (A), BLP-10_Cl (B), N2 uptake of BLP-1_Cl and BLP-10_Cl (C); and proposed structures of BLP-1_Cl (D, left) and BLP-10_Cl (D, right); Cl (green), N (blue), C (gray), and B (red).
Gas Sorption Analysis: Nitrogen sorption experiments at 77.3 K were performed on activated samples of BLP-1_Cl and BLP-10_Cl and produced Type I isotherms as classified by Brunauer, L. Deming, W. Deming, and Teller indicating microporous samples (Fig. 1C). Surface area calculations were performed on both products resulting in values exceeding that of analogous 2D polymers previously reported in literature.
We have used most of our PRF funds for the partial support of graduate and undergraduate students during the summer. Our research accumulated very significant data which was used to support a proposal to DOE entitled Design and Synthesis of Chemically and Electronically Tunable Nanoporous Organic Polymers for Use in Hydrogen Storage Applications. This proposal was funded for $570,000.00 (9/2009 to 8/2012) by DOE Basic Energy Sciences (BES) to expand the field of borazine-linked polymers and to investigate their performance in gas storage applications. Currently, we have one postdoctoral fellow, two graduates, and one undergraduate, working on this project. We are very thankful for the PRF for supporting our research which has already contributed significantly to the development of the PI's young research program as well as the continuing education of all students involved in the project.