Reports: GB1
48046-GB1 Synthesis of Indole Oligomers Via Iterative Suzuki Couplings
Indole-containing natural products have long been an inspiration to synthetic chemists. However, many chemists are unaware that one of the most visible natural products, melanin pigments in human skin, hair, and eyes, are indole-based materials. Eumelanin, the brown to black pigment in humans, is known to be composed of dihydroxyindole monomers; these are thought to form relatively short covalent oligomers that self-assemble into nanoparticles. The oligomers are composed of four to eight monomers and are heterogeneous in nature. Such oligomers are within the range of modern organic synthesis, similar to oligoarene foldamers. The ability to produce well-defined synthetic oligomers will advance our knowledge of this unusual biomaterial. This grant focuses on developing the synthetic methods necessary to generate indole and dihydroxyindole oligomers of relevance to eumelanin, particularly via iterative Suzuki couplings.
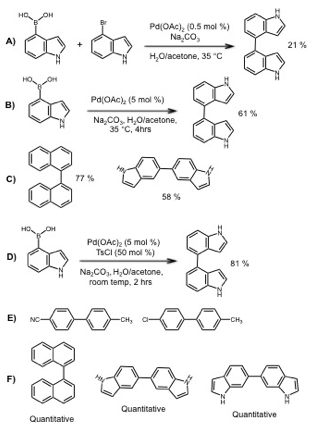
Palladium catalyzed cross-coupling of aryl boronic acids with aryl halides––the Suzuki-Miyaura reaction––is a central method for constructing aryl-aryl carbon bonds, and is increasingly being adapted for oligoarene synthesis. Indeed, palladium catalysis can be used to both install and couple aryl boronate groups. However, indole-indole Suzuki couplings are rare; thus, we began by examining indole dimerization. Prior to the initiation of PRF funding, we had investigated Zhang and co-workers' method1 for ligand-free Suzuki reactions. This method is notable in that the reactions can be open to air, making them operationally quite simple, and excellent yields were reported for biaryl products within an hour at 35 °C. In our hands, this method was inefficient for the desired coupling, providing 4-4' bi-indole in 21% yield from 4-bromoindole with 4-boronic acid indole (Figure 1A). However, when 4-bromoindole was serendipitously left out of the reaction mixture we observed palladium black precipitation, a hallmark of reactivity in such reactions. In fact, the desired 4-4' bi-indole was also present in the reaction mixture, presumably from the homocoupling of 4-boronic acid indole. Repeating the serendipitous reaction with an increased catalyst loading of 5 mol % Pd(OAc)2 provided 4-4'-bi-indole in 61% yield (Figure 1B).
|
X
|
Conditions as Shown Isolated Yield
|
Conditions as Shown + TsCl (50 mol %) Isolated Yield
|
|
-OMe
|
94 %
|
Quantitative
|
|
-Me
|
84 %
|
Quantitative
|
|
-Cl
|
97 %
|
80%
|
|
-F
|
31 %
|
Not Observed
|
|
-CN
|
32 %
|
60%
|
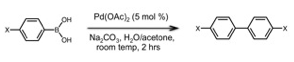
During the initial period of the grant, we have focused on optimization of this reaction. To explore the scope and limitations, we investigated a series of para-substituted aryl boronic acids, using 5 % mol Pd(OAc)2 and sodium carbonate in an aqueous/acetone solvent mixture (Table 1, Columm 2). The reactions were performed open to the air, at room temperature for two hours. High yields of the corresponding bi-aryl were obtained with electron-donating substituents, and, idiosyncratically, 4-chloro-aryl-boronic acid. Napthyl-, indole-, and other heteroaryl boronic acids were subjected to the same reaction conditions with mixed results. Successful substrates included 1-boronic acid napthelene, providing 1-1' bi-napthyl in 77% yield, and 5-boronic acid indole, providing 5-5' bi-indole in 58% yield (Figure 1C).
We sought to further optimize the reaction while retaining its operationally straightforward nature. In preliminary experiments, added quinones or copper salts as (co)-oxidants were not effective. Following a report from Kabalka,2 we included tosyl chloride (0.5 eq. relative to boron) in the reaction of 4-boronic acid indole under otherwise standard conditions, and obtained the 4-4' bi-indole in 81% yield (Figure 1D). The para-substituted aryl boronic acids had a different pattern of reactivity than without tosyl chloride, as seen by comparing Column 3 of Table 1 with Column 2. With the cyano- and chloro- substituents, 10 – 20 % of the heterodimeric product from desulfitative cross-coupling with tosyl chloride was observed (Figure 1E). Overall, the results suggest that tosyl chloride can oxidatively insert into an active palladium center under the reaction conditions. However, cross-coupling is disfavored under these conditions, so that tosyl chloride primarily acts as a co-oxidant and/or as a ligand for palladium during boronic acid homocoupling.
Indoles with boronic acid substituents at the 2-6 positions were subjected to the modified conditions, as well as other heteroaryl- and napthyl boronic acids (Figure 1F). 1-1' bi-napthyl was obtained in quantitative yield (compared to 77% without tosyl chloride). 5- and 6-boronic acid indoles provided 5-5' and 6-6' bi-indoles in quantitative yield (compared to 58 % without tosyl chloride for the 5-substution). Coupling by this method was less successful for indoles with the boronic acid substituent on the 5-membered ring, with substantial proteodeboronation under these conditions.
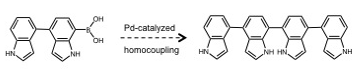
Homocoupling has largely been viewed as an undesired side-reaction in Suzuki cross-coupling, but with the recent explosion in the number of commercially available boronic acids, there is a growing interest in palladium-catalyzed homocoupling for symmetrical biaryl synthesis.2-4 Under the conditions described here, for many substrates yields are high, and the reaction is fast and operationally simple (air, water, room temperature). A manuscript on this work is in preparation with three undergraduate co-authors. With respect to indole oligomers of relevance to eumelanin, we are now in a position to incorporate homocoupling as a final step in our iterative syntheses, shown schematically in Figure 2. This is the focus of current efforts. Another recent development in the lab has been the purchase of a CEM Explorer 12 Hybrid microwave synthesizer (purchased with start-up funds). We are beginning to investigate the potential benefits of microwave reactions for the homocoupling reaction and other palladium-catalyzed reactions useful for the construction of indole oligomers.
References:
1. Lui, L.; Zhang, Y.; Xin, B. "Synthesis of Biaryls and Polyaryls by Ligand-Free Suzuki Reaction in Aqueous Phase," J. Org. Chem. 2006, 71, 3994-3997.
2. Kabalka, G. W.; Wang, L. "Lignandless palladium chloride-catalyzes homo-coupling of arylboronic acids in aqueous media," Tetrahedron Lett., 2002, 43, 3067-3068.
3. Adamo, C.; Amatore, C.; Ciofini, I.; Jutand, A.; Lakmini, H. "Mechanism of the Palladium-Catalyzed Homocoupling of Arylboronic Acids: Key Involvement of a Palladium Peroxo Complex." J. Am. Chem. Soc. 2006, 128, 6829-6836.
4. Yamamoto, Y. "Homocoupling of Arylboronic acids with a Catalyst System Consisting of a Palladium(II) N-Heterocyclic Carbene Complex and p-Benzoquinone," Synlett, 2007,1913-1916.