Reports: B3
48504-B3 Synthesis, Characterization, and Reactivity of Platinum/Ruthenium Heterometallic Complexes
Progress has been achieved in three distinct arms of the overall project studying how structural changes in platinum-ruthenium complexes influence reactivity with alcohols in atmospheric oxygen. Previous work establishes the procedure to synthesize the platinum rich heterometallic complex [(dppePt)2(m3-S)2Ru(PPh3)2Cl]Cl (dppe = 1,2-bis(diphenylphosphino)ethane) and that this complex does oxidize neopentyl alcohol with atmospheric oxygen. Three undergraduate students did research during academic terms and during the summer on the synthesis of heterometallic complexes. The majority of their progress was achieved during the summer.
One student, working toward the synthesis of the platinum rich complex [(dppePt)2(m3-S)Ru(PPh3)2Cl]Cl, optimized synthetic procedures for platinum-containing starting materials. The synthesis of the platinum monomer, dppePtCl2, has been refined and improved from a conventional synthesis reducing the time required for the reaction from 8 hours to 1 hour using a microwave reactor. The recrystallization in DMF is also quickly and successfully completed with a 3 minute cycle in the microwave and then precipitation with diethyl ether. Using a Biotage Initiator microwave synthesizer, isolated yields up to 71% have been achieved. Modifications of the synthetic procedure allow the synthesis of platinum monomers with other phosphine ligands such as the monodentate triphenylphosphine, and bidentate dppm or dppp. The modifications follow the changes found in the conventional synthesis for the complexes. For example, the optimized microwave synthesis for dppmPtCl2 uses water like the conventional synthesis does whereas the optimized microwave synthesis for dppePtCl2 does not, also like its conventional synthesis. The microwave conditions influence the ratio of cis versus trans isomers for (Ph3P)2PtCl2 based upon 31P NMR. The initial attempt to remove a sulfur atom from (dppe)2Pt2S2 by reductive desulfurization yielded a mixture of products that are being further investigated.
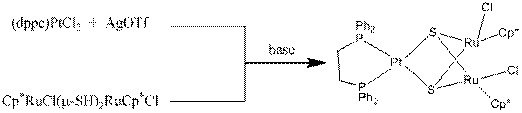
Another student is working toward the synthesis and characterization of the ruthenium rich complex (dppe)Pt(m3-S)2(RuCp*Cl)2. A procedure has been developed that leads to the isolation of a product whose 31P and 1H NMR spectra are consistent with the desired product.
Formation of by-product precipitates (AgCl and PSH+Cl-) during the synthesis also provides evidence that the reaction is proceeding towards the desired product. Attempts at growing crystals are underway.
Studies of the reactivity of the starting materials and related complexes with neopentyl alcohol are in progress. The reactivity studies are performed at a temperature of ~60°C in the presence of atmospheric oxygen and are evaluated by 1H NMR using an internal standard. The starting materials for the ruthenium-rich complex, dppePtCl2 and (Cp*ClRu)2(m2-SH)2, do not show any reactivity after 72 hours. Related materials are reactive with neopentyl alcohol, with [(Cp*ClRu)2(m2-Cl)2]n having a turnover of 2.61 at the 72 hour mark versus (Cp*ClRu)4 which has a turnover of 3.97 at the 72 hour mark for the formation of the aldehyde product. Additional related complexes are currently being synthesized for a greater breadth of complexes for reactivity studies.
The third student is working toward the synthesis of additional Pt-Ru heterometallic complexes with equal numbers of platinum and ruthenium atoms. The platinum monomer (Ph3P)2Pt(SH)2 has been synthesized as a starting material with the presence of the SH being confirmed by IR. Reaction of (Ph3P)2Pt(SH)2 and (Cp*ClRu)4 results in a dark brown product. This dark brown product does not contain an SH moiety based upon the lack of a SH stretch in the IR. The 31P NMR shows a single phosphorus peak with platinum satellites. The chemical shift and J coupling match the starting material. The great difference in color of the product from the starting (Ph3P)2Pt(SH)2 after multiple washings and recrystallization indicates that this product is of interest despite the great similarity in the 31P NMR spectra of the starting material and product. One possible identity for the product is [(Ph3P)2Pt(m2-S)2RuCp*Cl]2-.
Funds from the grant made it possible for three undergraduate students to contribute to the project. A solvent purification system was purchased and installed which saves time, provides a cleaner solvent, and is safer than distillation from active reagents. An undergraduate researcher presented a poster titled Investigating the Reactivity of a Series of Ruthenium and Platinum Complexes by Comparison of their Ability to Oxidize Neopentyl Alcohol at the March 2009 American Chemical Society meeting in Salt Lake City.