Reports: G3
48170-G3 Synthesis of Polymers for Photovoltaic Cells by Cross-Coupling Reactions Mediated by NHC-Palladium Complexes (NHC = N-Heterocyclic Carbene)
The use of
polythiophenes as conducting polymers has attracted intense interest for
applications in many organic electronic devices, such as field-effect
transistors (FETs), thin-film transistor (
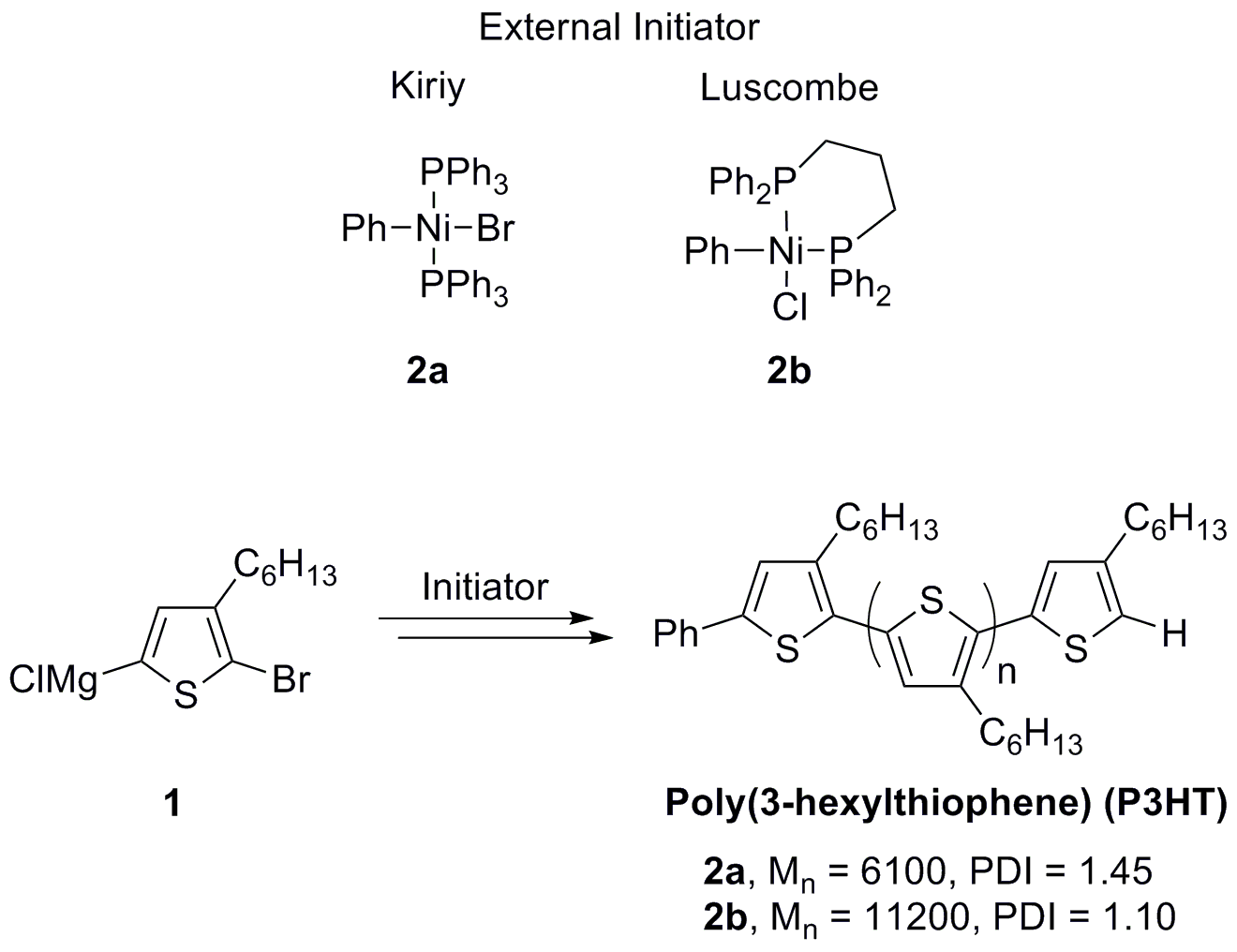
Scheme 1. Thiophene polymerization using an external initiator
In recent years, N‑heterocyclic carbenes (NHC) have been extensively studied and their use as ligands for transition-metal complexes has allowed significant improvement of many catalytic reactions when compared with analogous phosphine-systems.8 Thus, we envisaged that the replacement of phosphine ligand with an NHC could improve this polymerization process. Interestingly, to our knowledge, there are no precedents for the use of NHC-bearing complexes for the synthesis of polythiophenes.
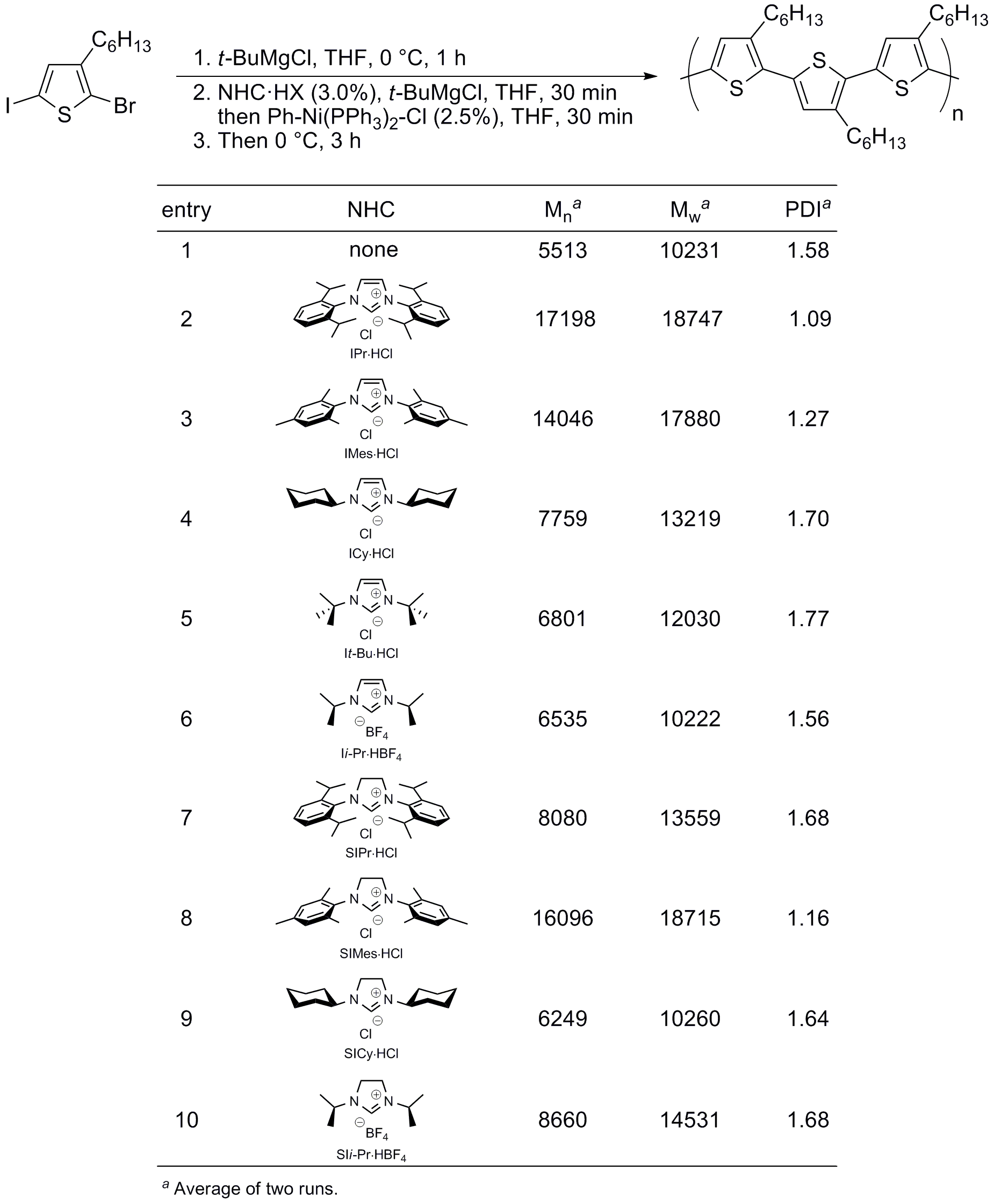
Our initial goal was the development of (NHC)Pd systems for the polymerization of thiophenes using Suzuki-Miyaura cross-coupling reactions.9 We wanted to address two main limitations of the GRIM process: low functional group tolerance (hence research in the area mainly focuses on polyalkylthiophene frameworks) and the need of preparing the Grignard reagent in situ, which requires the stoichiometry of the reaction to be precisely controlled in order to achieve high molar mass material. In order to get a preliminary confirmation of the positive effect of the use of NHC as ligands for the synthesis of polythiophenes, we decided first to test our premise in a GRIM (Ni-catalyzed) polymerization, using a modification of Kiriy's conditions.7a Different parameters (catalysts loading, temperature and reaction time) were tested in order to determine the optimized conditions. The outcome of this preliminary study is that results are optimum using Ph‑Ni(PPh3)2‑Br as the pre-catalyst (2.5 mol%) in the presence of a small excess of the imidazolium salt (precursor of the NHC) at 0 °C for 3 hours. Under this set of experimental conditions, different NHC were tested and the results are summarized in REF _Ref241399891 \h Table 1. We were delighted to find that the use of 1,3‑bis(2,6‑diisopropylphenyl)imidazol-2‑ylidene chloride (IPr·HCl) afforded high molecular weight polymers with one of the lowest values of polydispersity reported to date for this molecular weight range (1.09).
Interestingly, 1H NMR analysis of the resulting polymers indicates that under our reaction conditions the phenyl fragment is not attached to the resulting polymer chains. We presume that the coordination of the NHC to the metal induces the reductive elimination of phenyl bromide prior to the oxidative addition of the first monomer.
Currently, we are carrying out experiments to determine the catalytic active species and the fate of the phenyl bromide fragment. Findings on this process will also allow us to design a well-defined NHC-bearing complex to act as a pre-catalyst for these polymerizations. At the same time, we are synthesizing chlorothiophene monomers to attempt the first polymerization of that type of substrates. We are also starting to have a look at new thiophene scaffolds with other functional groups different than alkylic chains.
Table SEQ Table \* ARABIC 1. (NHC)‑Ni Catalyzed Thiophene Derivative Polymerization
REFERENCES
[1]. Jeffries-El, M.; McCullough, R. D. in Handbook of Conducting Polymers, 3rd ed.; Skotheim, T. A.; Reynolds, J. R.; Eds.; CRC Press Inc Taylor & Francis Group: Boca Raton, 2007, Chapter 9.
2. Günes, S.; Neugebauer, H.; Sariciftci, N. S. Chem. Rev. 2007, 107, 1324‑1338.
3. For the McCullough Method, see: (a) McCullough, R. D.; Lowe, R. D. J. Chem. Soc., Chem. Commun. 1992, 70-72. (b) McCullough, R. D.; Lowe, R. D.; Jayaraman, M.; Anderson, D. L. J. Org. Chem. 1993, 58, 904-912.
4. For the Rieke Method, see: (a) Chen, T.-A.; Rieke R. D. J. Am. Chem. Soc. 1992, 114, 10087‑10088. (b) Chen, T.-A.; O'Brien, R. A.; Rieke, R. D. Macromolecules 1993, 26, 3462‑3463. (c) Chen, T.-A.; Wu, X.; Rieke R. D. J. Am. Chem. Soc. 1995, 117, 233‑244.
5. For the GRIM methods, see: (a) Loewe, R. S.; Khersonsky, S. M.; McCullough R. D. Adv. Mater. 1999, 11, 250‑253. (b) Loewe, R. S.; Ewbank, P. C.; Liu, J.; Zhai, L.; McCullough, R. D. Macromolecules 2001, 34, 4324‑4333.
6. (a) Yokoyama, A.; Miyakoshi, R.; Yokozawa, T. Macromolecules 2004, 37, 1169‑1171. (b) Sheina, E. E.; Liu, J.; Iovu, M. C.; Laird, D. W.; McCullough, R. D. Macromolecules 2004, 37, 3526‑3528. (c) Iovu, M. C.; Sheina, E. E.; Gil, R. R.; McCullough, R. D. Macromolecules 2005, 38, 8649‑8656. (d) Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. J. Am. Chem. Soc. 2005, 127, 17542‑17547. (e) Miyakoshi, R.; Yokoyama, A.; Yokozawa, T. J. Polym. Sci., Part A 2008, 46, 753‑765. (f) Yokozawa, T.; Yokoyama, A. Chem. Rev. 2009, DOI: 10.1021/cr900041c.
7. (a) Beryozkina, T.; Senkovskyy, V.; Kaul, E.; Kiriy, A. Macromolecules 2008, 41, 7817‑7823. (b) Doubina, N.; Ho, A.; Jen, A. K.-Y.; Luscombe, C. K. Macromolecules 2009, DOI: 10.1021/ma901410k. (c) Bronstein, H. A.; Luscombe, C. K. J. Am. Chem. Soc. 2009, DOI: 10.1021/ja9054977.
8. (a) Hermann, W. A. Angew. Chem., Int. Ed. 2002, 41, 1290‑1309. (b) Nolan, S. P., Ed. N‑Heterocyclic Carbenes in Synthesis; Wiley‑VCH: Weinheim, Germany, 2006. (c) Glorius, F., Ed. N‑Heterocyclic Carbenes in Transition Metal Catalysis; Topics in Organometallic Chemistry, Vol 21; Springer‑Verlag, Berlin/Heidelberg, Germany 2007. (d) Díez-González, S.; Nolan, S. P. Coord. Chem. Rev. 2007, 251, 874‑883.
9. Miyaura, N. Organoboron Compounds. Top. Curr. Chem. 2002, 219, 11-59.