Our observation that all of the
applications of the [Ru3O(O2CR)6(L)3]n+
class of compounds had been carried out with the acetate species prompted us to
pursue a general strategy for preparing other carboxylate
species in this system. Our experience,
combined with the evidence from the literature, is that the largest challenge
is the separation of the ruthenium-containing products from their by-product
salts. We have successfully used the
synthetic strategy below to prepare and characterize two butyrate species,1 [Ru3O(O2CCH(CH3)2)6(py)3] and [Ru3O(O2CCH2CH2CH3)6(py)3]
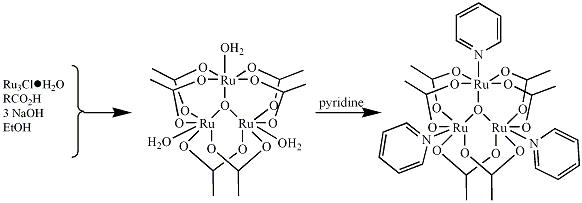
Figure 1. General
strategy for the synthesis of basic ruthenium carboxylates
Our efforts to optimize the
synthesis have correlated with the report by Toma and
coworkers,2 that this apparently simple
system is actually extremely sensitive to pH, even when carried out in organic
solvents. By using a generous amount of
acid in the first step and by minimizing the pyridine in the second step, the
desired blue complexes may be produced reliably. Our experiments have determined that in the
presence of less acid in the first step and more base in the second, that a
green product, hypothesized to be the neutral complex, [Ru3O(O2CCH2CH2CH3)6(OH)(py)2], results from the deprotonation of one axial water ligand. The resulting axial hydroxide blocks ligand exchange with pyridine. We are currently refining the purification to
isolate solid samples of this product for complete characterization.
The metal oxidation states of Ru3III,III,III necessitate an overall paramagnetic system,
since the triangular metal architecture precludes complete spin pairing. Room
temperature magnetic susceptibility data indicate a value less than one
unpaired electron and preliminary analysis of SQUID data by our collaborator
Ted Barnes at the University of Tennessee also
suggests that the system is not a simple paramagnet.
The paramagnetism
of the clusters has an observable impact on the 1H NMR
spectrum. Resonances due to protons closer
to the metal centers display significant relaxation; splitting patterns are
more well resolved for protons at increasing distance from the metals so that
the para proton on the axial pyridine ligand gives rise to a well-resolved triplet. Somewhat unexpectedly, the ortho pyridine proton is not only relaxed, but also shifts
substantially upfield to near 1.0 ppm
depending on the temperature. The
identity of this proton resonance has been confirmed through the use of 1H
COSY NMR.
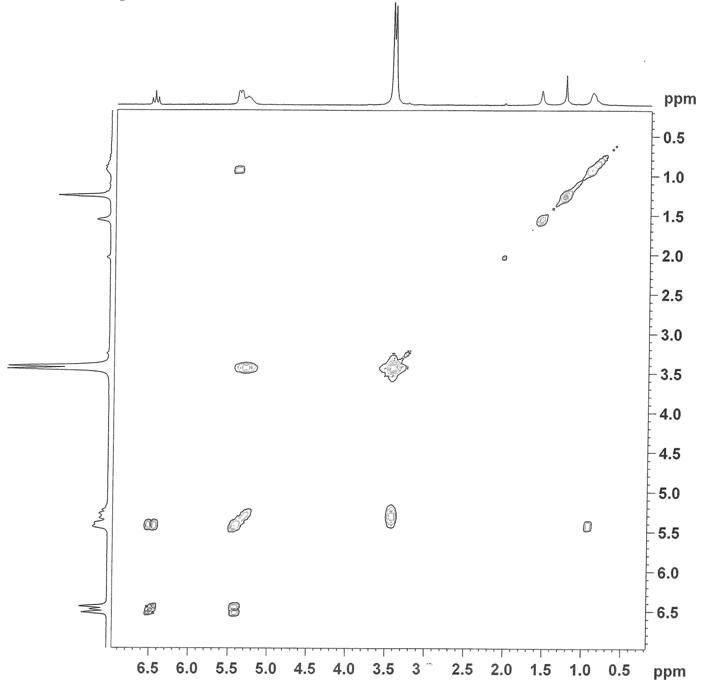
Figure 2. COSY 1H
NMR [Ru3O(O2CCH(CH3)2)6(py)3](PF6)
The Department of Chemistry at the
University of Hartford places high priority on
undergraduate research experiences for our students, and this PRF grant has had
a significant impact on the progress of both me and my students. Megan Burak was
unable to work for her full commitment in summer 2009 due to personal reasons,
but she still benefited from the structure and accountability of her research
experience. She is an innately bright
student, and her performance level rises when she has personal ownership of her
learning and is required to analyze her own data to understand the various
processes that are involved. Ali Svenson, the newest student to work on this project, had
completed only two semesters of general chemistry when she started working on
her PRF-funded project. Regular group
meetings and advisor/student conferences focusing on the both the strategies
for synthesis and purification as well as on the background for the various
characterization methods inspired her to do a considerable amount of self-study
to learn the background material. She is
currently taking her first semester of organic chemistry and recently
commented, After research, organic is pretty easy. From my perspective as PI, the PRF grant has
had several impacts. Although the
manuscript submitted to Dalton
Transactions mentioned in last year's report was not accepted, I plan to
reshape it and submit it to a different journal in the upcoming year. I also mentioned in last year's report that I
submitted my application for promotion to Full Professor in September
2008. At the University of Hartford,
promotion to full professor is based in part on demonstrating sustained and
distinguished performance in the traditional scholarship of discovery. The University approved my promotion in
January 2009, and I am grateful to the support of PRF as part of achieving this
professional goal.
References
1.
C. C. Pink,* N. L. Saad,* Mugge,
A. M.*; J. M. Schlough,* Svenson,
A.*; J. L. Eglin, Pence, L. E., manuscript in preparation.
2.
Nunes, G. S.; Alexiou,
A. D. P.; Araki, K.; Formiga, A. L. B.; Rocha, R. C.;
Toma, H. E. Eur. J. Inorg. Chem. 2006,
1487-1495.






