Reports: GB3
48039-GB3 Novel Organic-Inorganic Hybrid Cage Architectures Incorporating Azacrown Ethers and 1,3,5-Triazine Scaffolds
In the first year of the grant we have explored the possibility to prepare neutral Metal-Organic-Rotaxane-Frameworks.
The last decades witnessed an increased interest in the synthesis and characterization of molecular machines and devices. Mechanically interlocked molecules, such as rotaxanes and catenanes have become the representative class of active components in devices at the molecular level. Rotaxanes (systems in which one or more rings are threaded by one or more axles) are particularly promising since their topology allows the macrocyclic component to undergo a reversible translational motion upon excitation with an appropriate stimulus, thus behaving as a molecular shuttle or switch.
An important research direction in this field is the synthesis of Metal-Organic-Rotaxane-Frameworks (MORFs), which are a particular class of Metal-Organic-Frameworks (MOFs) with rotaxanes functioning as one of the linkers between the metal nodes. These architectures will ultimately provide a simple and modular method to create a crystalline lattice comprising of dynamic molecular components. While several examples of MORFs are known, they lack the necessary features to allow molecular motion. Ideally, such a MORF should be built on a two (or more) station rotaxane and should consist of a robust, porous, and overall neutral crystalline material, free of counter-ions and solvent molecules to allow the wheel to undergo the translational movement between the rotaxane stations. An elegant approach toward this goal is used by Loeb, employing zwitterionic rotaxanes as ligands, and connecting them to neutral, metal-containing nodes.
We have started to explore a new and different approach,using anionic rotaxanes as ligands that would produce, after coordination to cationic metal-containing nodes, a neutral (counter-ion free) network.
In our efforts to build an anionic rotaxane, we have focused our attention on the neutral naphthalenediimide/15DN38C10 system, since the interactions between these electron-poor / electron-rich have been successfully used in directing the formation of mechanically interlocked systems. We have also sought to incorporate in the structure of these rotaxanes groups with donor capabilities such as carboxylic acids and soft ligands such as the dithiophosphonate moiety because of their rich coordination chemistry and their propensity to form multidimensional supramolecular arrays via secondary bonds when suitable soft metals are used as nodes, therefore increasing the possibility of the self-assembling of the basic metallorotaxanes into networks of higher dimensionality.
The dicarboxylic functionalized naphthalenediimide axles were synthesized by reacting the 1,4,5,8-naphthalene-tetracarboxylic dianhydride with two equivalents of 4-amino-butanoic acid in DMF, then deprotonating the resulting acid either with NH4OH or NMe4OH. The formation of the pseudorotaxane by the template-directed synthesis from their components, by mixing them in the required 1:1 stoichiometry was achieved in an acetone-water solvent system.
The dithiophosphonate functionalized naphthalenediimide axles were synthesized by reacting the 1,4,5,8-naphthalene-tetracarboxylic dianhydride with two equivalents of either 2-aminoethanol or 2-(2-aminoethoxy)ethanol in DMF, then reacting the resulting diols with 1,3,2,4-dithiadiphosphetane-2,4-bis(4-methoxyphenyl)-2,4-disulfide (Lawesson's Reagent) in benzene. The ditopic ammonium salts NDI[(CH2CH2O)n(An)PS2NH4]2 (n=1 or 2, An=anisole) were then precipitated as orange solids by passing a stream of dry gaseous NH3 through the cold benzene solution of the dithiophosphonic acids.
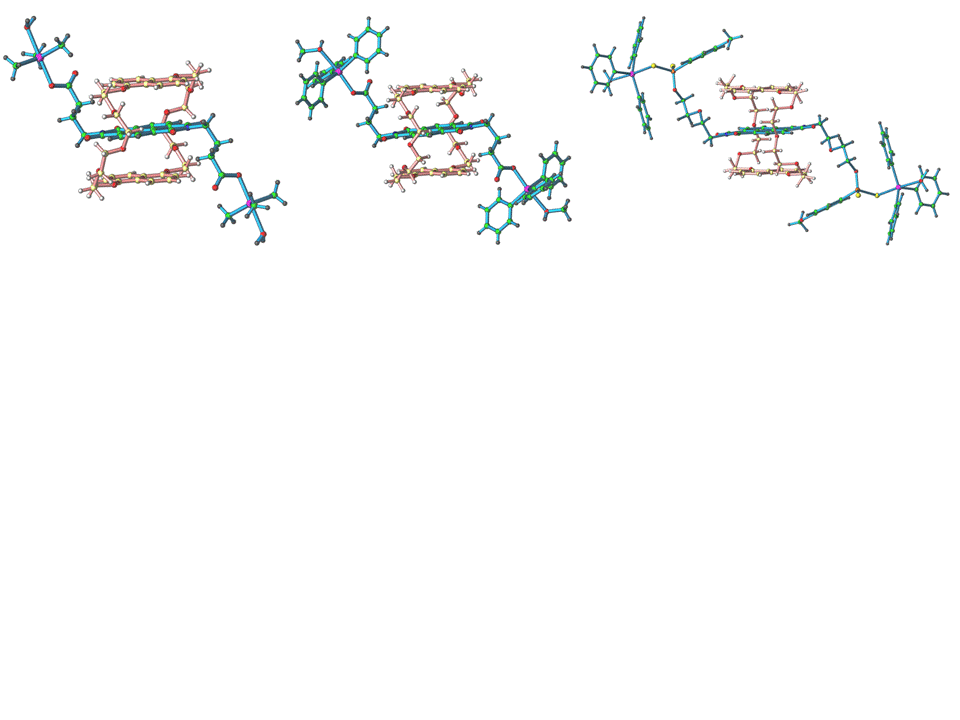
The reaction of these ligands to Ph3SnCl and Me3SnCl produced neutral discrete rotaxanes, which were characterized by NMR and UV-Vis spectroscopy (to determine their association constants Ka) and by X-ray diffraction studies. Figure 1 shows the structure of three such rotaxanes, all built on the naphthalenediimide/15DN38C10 recognition motif. Their general structural features are identical: a 15DN35C10 wheel threaded onto the corresponding axle, with the side arms of the axles oriented in a trans orientation with respect to the central NDI moiety, and having their coordinate vectors oriented in opposite directions.
Figure 1. Molecular structures of naphtalenediimide based rotaxanes with organometallic stoppers.