Reports: AC1
47306-AC1 Diels-Alder Cycloaddition Reactions with Dihapto-Coordinated Pyridines
Our original study supported by the PRF concerned the promotion of Diels-Alder reactions with pyridines acting as dienophiles. These reactions are promoted by the ¹ basic tungsten complex {TpW(NO)(PMe3)}, which binds across C3 and C4 (h2), thus rendering the pyridine chemically similar to a 2-azadiene. This study was completed ahead of schedule, was summarized in last year's progress report, and was recently published. Over this past year, our focus has shifted to uncovering new reaction sequences for tungsten-pyridine complexes. Progress in three areas is described below.
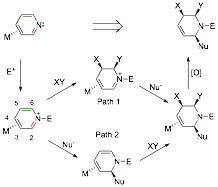
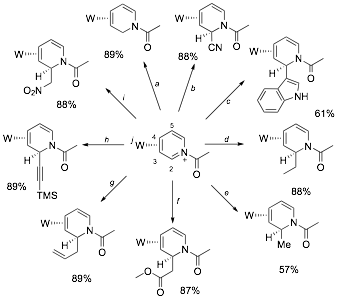
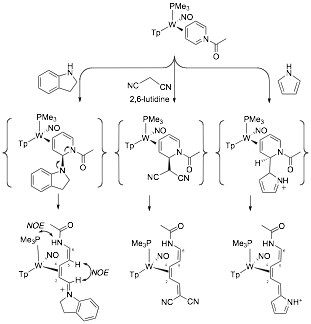
1. Stereo- and regiospecific nucleophilic addition to C2 of pyridinium complexes. While the chemistry of arene ¹ complexes has been thoroughly explored, comparatively little is known about the chemistry of ¹-bound heterocycles. Consider the pyridine complex TpW(NO)(PMe3)(h2-pyridine) (1), in which the heterocycle is coordinated by W across C3 and C4. As a consequence of metal-to-ligand ¹ backbonding, the nucleophilicity at nitrogen is enhanced, providing a route to stabilized pyridinium complexes. Such complexes were recently shown to undergo 5,6-dialkoxylation without compromising the coordinating metal, and the subsequent addition of a nucleophile at C2 led to several novel D3-piperidines (see above; Path 1). The goal of the this study was to explore the complimentary reaction sequence (Path 2): nucleophilic addition at C2 followed by elaboration at C5 and C6. We found that with C3 and C4 of pyridine coordinated by the dearomatization agent {TpW(NO)(PMe3)}, the heterocyclic nitrogen becomes 6-7 orders of magnitude more basic. This nitrogen could be acylated or alkylated forming stable h2-pyridinium complexes. The N-acetylated variant readily undergoes regio- and stereoselective nucleophilic addition: Addition occurs exclusively at C2, anti to the tungsten. This reaction occurs with a remarkably broad range of nucleophiles including enolates, acetylides, alkyllithiums, alkylzincs, hydrides, cyanide, and nitrogenous heterocycles (indole, pyrrole, etc). These results have just been accepted for publication.
2. Elaboration of dihydropyridine complexes into D3-piperidines.
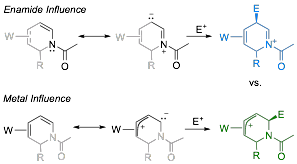
With a broad range of 2-substituted h2-1,2-dihydropyridine complexes in hand, we endeavored to functionalize the remaining double bond at C5-C6. Enamides, like enamines, polarize the alkene bond such that electrophiles normally add to the b carbon. However, studies of h2-coordinated 1,3-dienes show that electrophilic addition typically occurs at the uncoordinated terminal alkene carbon. Thus, 1,2-dihydropyridine complexes with the metal at C3,C4 present an interesting regiochemical question, pitting the electron donation of the amide nitrogen against that of the ¹ basic tungsten complex. Here we find that protonation occurs exclusively at C6 (an umpolung), resulting in a ¹ allyl complex.
This allyl
species was found to be highly asymmetric, both in terms of its electronic
character and its chemical structure.
The bond lengths of tungsten to the two terminal allyl carbons differ by
almost 0.4 , with C3 taking on considerable free carbocation character. Correspondingly, C3 of the pyridine
ring is readily is attacked by a range of nucleophiles generating
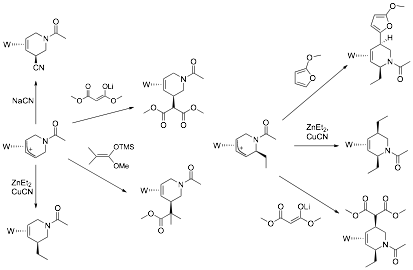
5,6-disubstituted D3-piperidine
complexes. In certain cases, steric factors direct the second nucleophilic
addition to C5 of the pyridine ring, resulting in 2,5-disubstituted D3-piperidines. These observations of nucleophilic
addition to C3 or C5 of a pyridine ring system represents an umpolung of the
natural chemical behavior of this heterocycle allowing for the preparation of
unique substitution patterns not easily available by conventional methodsspan>
3. Ring-opening of the pyridine core promoted by tungsten.
Cyanines and merocyanines,
polyene systems with an electron donor group at one terminus and a withdrawing
group at the other, are widely studied not only in the historical context of
the dye industry, but for their non linear optical and solvatochromism
properties, and their potential uses in laser technology, data storage, photosensitizers, and phototheraputics. In the course of
investigating the reactivity of h2-coordinated pyridinium complexes of
tungsten, we came across an unexpected reaction pathway in which the bound
pyridinium ligand, upon treatment with certain nucleophiles, spontaneously
underwent ring scission, cleanly forming a new type of "metallocyanine".