Reports: B7
45134-B7 Competition between Mesogens in Pyridone-Based Supramolecular Calamitic, Banana, and Discotic Liquid Crystals
In the first year, Justin Kumpfer worked on the synthesis of pyridone-based esters. It was found that while the substitution chemistry of pyridones was difficult, the species were capable of reacting through nucleophilic acyl substitutions. Metal-based coupling reactions proved problematic, as the pyridine functionality chelated the metal from the catalyst, rendering the reaction inert. These results provided the groundwork for the rest of the project.
In the second year, David Witte synthesized a series of bis-functionalized pyridone terminated esters. Supramolecular polymers produced flexible, long-lived fibers pulled from the melt, but interestingly produced only a frustrated nematic phase observable only upon crash cooling the isotropic melt in liquid nitrogen. It is believed that the nematic phase could only be captured in this dramatic fashion because the overall structure of the assembled pyridone species would be too irregular to effectively form a mesogenic phase.
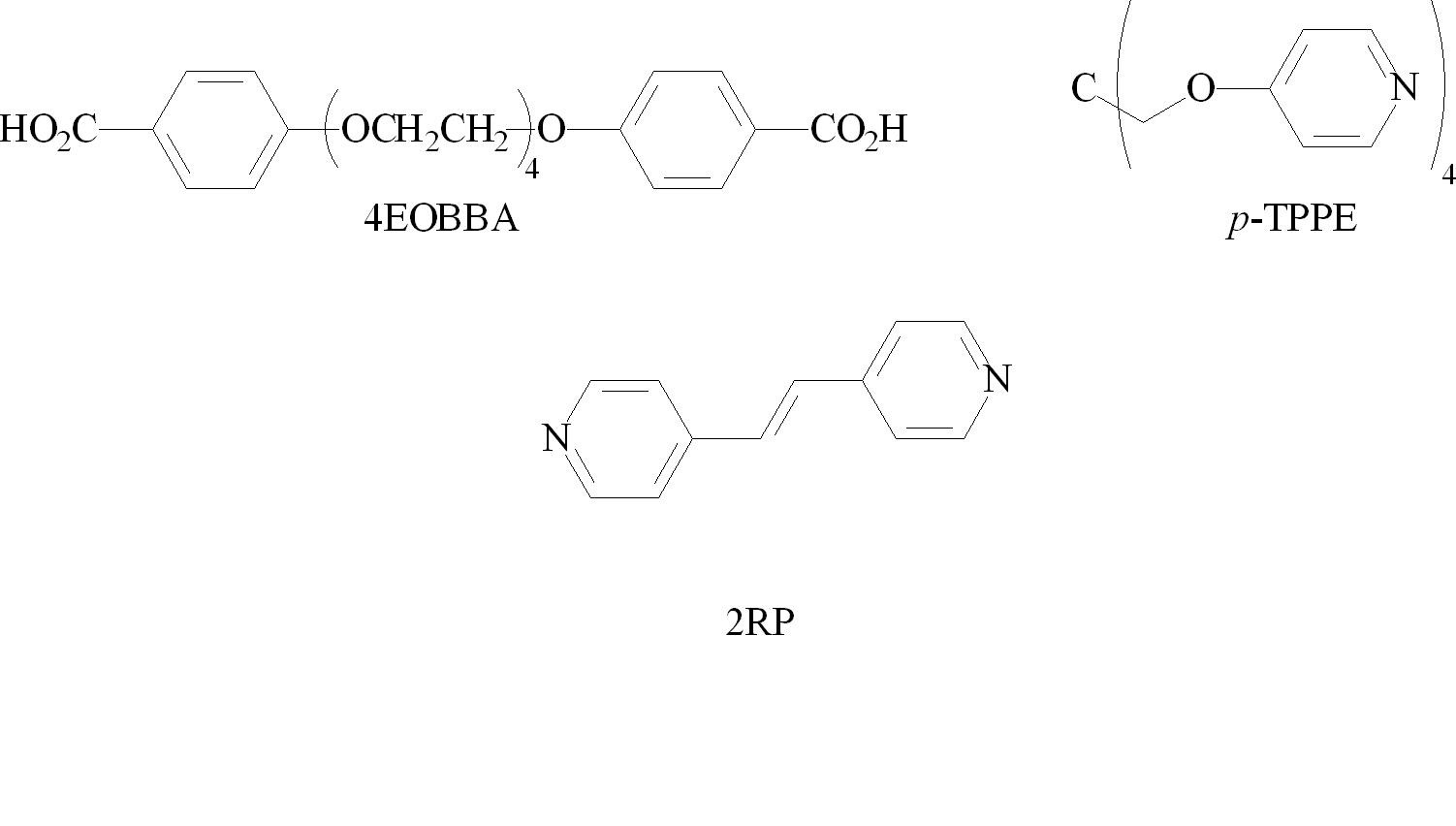
Also in the second year, a side project funded by this grant was carried out by Jason Greuel involving the investigation of the liquid crystalline properties of supramolecular networks. We observed the formation of a series of mesogenic networks with increasing netpoint inclusion. The mesogenic portions of these systems arise from a hydrogen bonded interaction between a bisbenzoic acid (tetraethyleneglycoxy-bis-4-benzoic acid, 4EOBBA), a rigid bispyridyl system (1,2-bis(4-pyridyl) ethylene), and a tetrafunctionalized non-mesogenic networking agent (tetrakis-4-pyrixyloxy methane, p-TPPE), which is used to introduce a network and disrupt liquid crystallinity. All the materials uses in this study are outlined in Figure 1. The networking agent was added in increasing concentrations to the system. A smectic phase was observed in concentrations up to 8.5% of networking agent. The nematic phase was observed until concentrations up to 22.5%, which displayed a frustrated nematic phase, barely forming before crystallizing. At 25% and above, only melting and crystallization behavior was observed. It was curious to note that the cut-off for any liquid crystallinity (22.5%) which correspons closely with the tetra-functionalized nature of the crosslinking agent. This phenomenon may arise from a statistical correlation, where at 25%, one of the benzoic acid components would always be hydrogen bonded with one of the networking species, inhibiting the formation of the hydrogen bond with the rigid 2RP species, and therefore inhibiting the formation of the hydrogen bond.
A further study involved annealing these networks in the mesophase and observing a premature onset of crystallization. This onset rose to higher and higher temperatures with increasing netpoint inclusion. This phenomenon was not observed in small molecule covalent or supramolecular liquid crystals. The first study has produced a paper which will soon be submitted to Liquid Crystals, and the second manuscript is in development. Both will have two undergraduate authors.
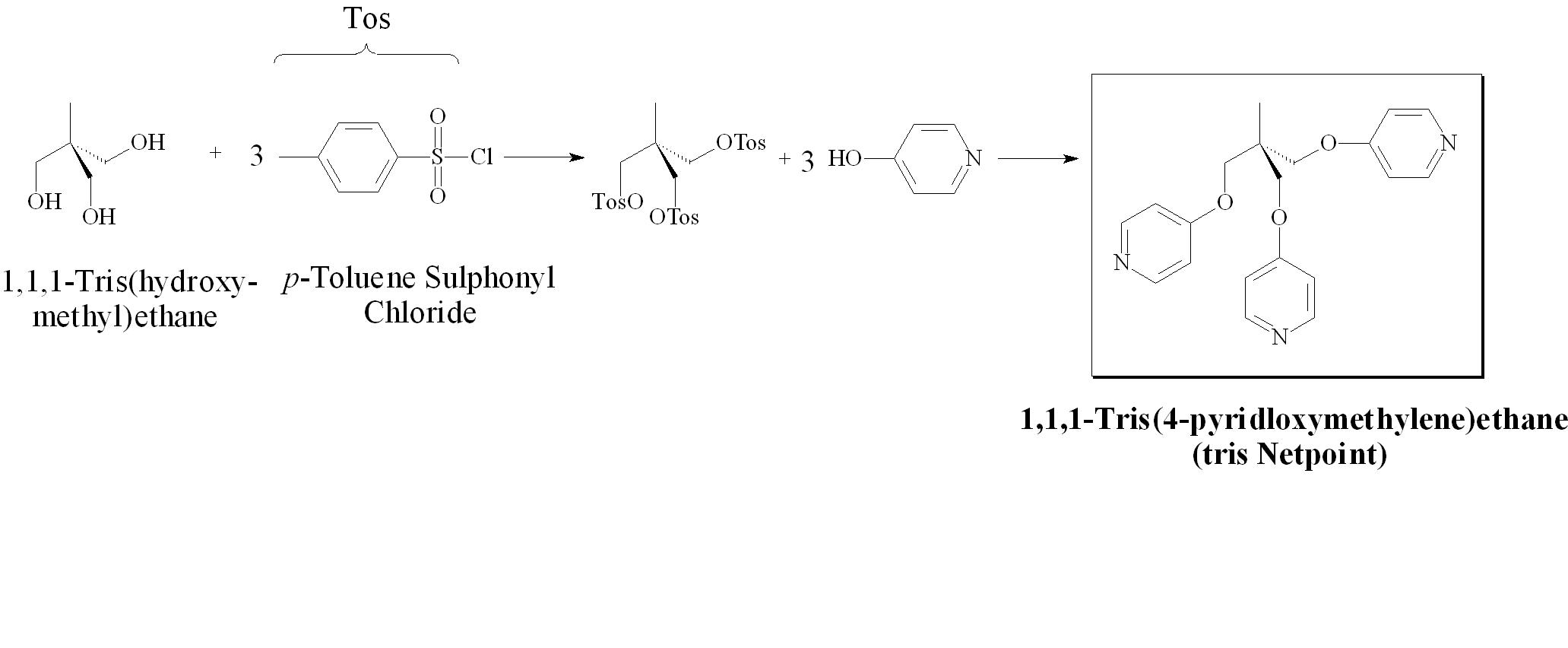
As the pyridone project seemed to be at an end, producing systems barely mesogenic in nature, further investigation was carried out on the networked systems. In order to determine if the statistical correlation between the functionalty of the networking agent and the disappearance of the liquid crystalline phase exists, a tris-functionalized networking agent was synthesized as outlined in Figure 2. The networks so created displayed smectic phases until 15% concentration, and a complete destruction of mesogenicity (also a frustrated nematic phase) at 32%. The results of this work are being compiled into a publication which will have two undergraduate authors and will be submitted to Liquid Crystals.
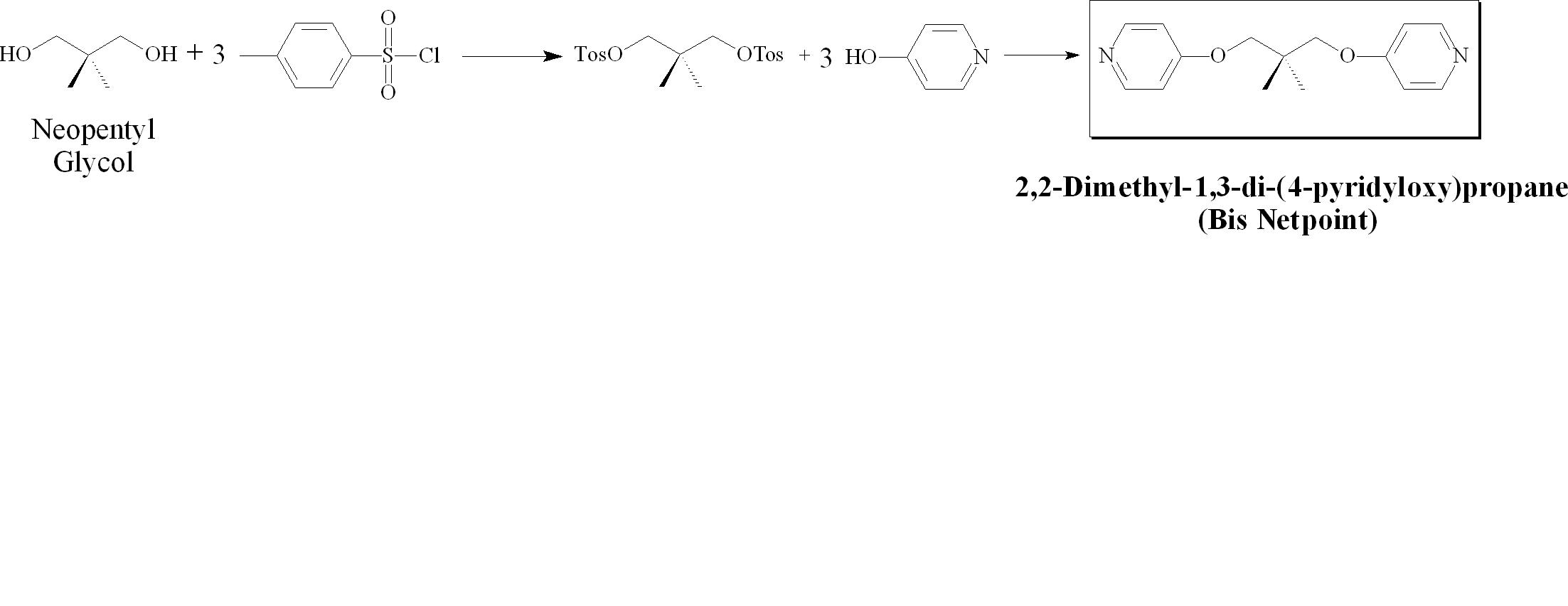
As a continuation of this work, a non-mesogenic bispyridyl system will be synthesized- as detailed in Figure 3. This species will be doped into the linear liquid crystalline polymer system to determine if the same effect takes place in a non-networked system. Synthetic work has already been undertaken and the analysis should be forthcoming shortly.




